The Importance of the Cold Chain Logistics in the Pandemic: The Transport of Covid-19 Vaccines by Mesut Selamoglu* in Open Access Journal of Biogeneric Science and Research
ABSTRACT
Pharmaceutical and medical products play a major role in the cold chain and its logistics, especially in temperature-sensitive products. Cold chain logistics are referred to as supply chain systems consisting of series of protocols including multi-level processing, transportation, storage, distribution and retail sale of products. All these procedures are indispensable to maintain a temperature-controlled environment for the pharmaceutical products such as drugs vaccines and to minimize deterioration and maintain quality standards. Therefore, with advancement in technologies in the industrial sector, cold chain logistics have made remarkable progress.
Keywords: Pandemic, Vaccines, Covid-19, Logistics, Cold chain, Cold chain logistics, Smart health technology
Introduction
Human history has witnessed many pandemics and significant impacts caused by these pandemics on health, economy and even global security. Such a pandemic situation has infected millions of people, caused massive diseases outbreak and deaths. Pandemics also threaten global security which directly influences economic stability and lives. The mortality and morbidity rates can be controlled by a prompt, efficient and effective emergency response to reduce long term social and economic impacts. The term ‘Pandemic’ is referred to the outbreak of a fatal disease at a global level irrespective of regions and climates such as the Black Death, HIV-AIDS and SARS and plague. In the 21st century, infections of avian influenza (bird flu) and SARS emerged and they cause pandemic which arose from Asia and caused millions of death and infected people all around the world. The emergence of new and virulent viral strains cause global pandemics and the spread of such infectious viral diseases is easy. Such infectious diseases lead to high rates of transmission, mortality and morbidity as the human population do not show immunity against the virus [1].
The outbreak of infectious diseases spreads very quickly and can cross borders very easily, which threatens not only economies but also the regional stabilities as was witnessed in the pandemic and epidemic of H1N1, H5N1, HIV and SARS. Such health emergencies caused by infectious diseases threaten public health as well as global stability. During December 2019, there were many reports of pneumonia type incidences caused by some unidentified viral strain outbroke Wuhan, China. The disease clinically resembled some new type of viral pneumonia and Flu. After the isolation of the virus and several analyses of its genomic sequence, a novel strain of coronavirus was identified which was designated as ‘severe acute respiratory syndrome-Related Coronavirus-2’ or SARS-CoV-2. The respiratory diseases were caused by this newly discovered ‘SARS-CoV-2’ which was later declared as ‘Coronavirus disease 2019’ (Covid-19) by World Health Organisation [2-4]. The virus originated from bats and then transmitted to human beings. This Covid-19 pandemic has been threatening human health all around the world and its chain is not yet been broken as humans are spreading it. This pandemic situation has lime lighted the worth of laboratory medicines. The clinicians, laboratories and medical scientists have played a very critical part and responded very promptly to this health emergency of SARS-CoV-2. Currently, the laboratory medicine services are required to take care of the rapid diagnosis and medical solution of this viral infection, serological and biochemical monitoring of infected the hospitalized patients and epidemiological surveillance. The immune responses of post-infection patients are detected by antibody assays against pathogens. Serological investigations are pivotal in determining the efficiency of vaccines and also in evaluating the immune response of patients. Especially, the federal drug authority takes an average of 12 years to approve a drug or vaccine for any disease. However, there are great efforts worldwide on the developing of vaccines against the Covid-19. Therefore, there are 5 different platforms for Covid-19 vaccines and about 16 different vaccines developed. The platforms and vaccines in each platform are as follows [5]:
- RNA based vaccine eg Pfizer, Moderna, CureVac G
- Viral Vector (adenovirus-non-replicating) eg AstraZeneca, CanSino, Sputnik, Janssen
- Inactivated Virus eg Sinopharm, Sinovac etc.
- Protein subsunit eg Novavax
- DNA based Vaccine eg Zydus Cadila
There was rapid progress and scientific efforts to develop vaccines for Covid-19 and from initial trials to the approval of vaccines the hopes were alive to break this chain of virus and stop the pandemic. The global distribution of the coronavirus vaccines required a huge setup of their transport and the logistic challenges in air transportation are fundamental. This present paper will encompass all key considerations regarding vaccination and its role in recovering international travels. The worldwide transportation of Covid-19 vaccines requires some stringent and safe means. After successful trials, the Covid-19 vaccines enlightened the hopes all around the world to get rid of the pandemic situation and returning to the normality of life. The global transportation of vaccines require international travels and this process must be standardized and consistent to minimize complexities and wastage of doses. Moreover, a clear roadmap plan is needed to implement and manage the vaccination process, especially during roll-out periods of different countries which are going at a different pace to access vaccine supplies. This is very crucial to maintain the vaccination process while there is an overlap between testing and vaccination [6]. The transportation and handling of life-saving pharmaceutical products demand stringent and careful handling conditions. During the transportation of potential medicines may lose their potency and become ineffective. The most critical issue while transporting pharmaceutical products is the maintenance of quality which cannot be compromised. The impacts of logistic constraints and their strategies are mitigated by dealing with pharmaceutical logistics. The operational challenges of transporting vaccines across the borders are linked to the standardized prerequisites to be fulfilled. The training of transportation staff is highly necessary so as their prior knowledgeability about the issue. The risk assessment and reviewing to adjust any risk is the primary requirement that has to be dedicated to the types of equipment and infrastructure [7].
There are different economic and technical indicators aimed at ensuring product control due to the inability to restore quality losses. The final quality of the product is evaluated together with the time, temperature and tolerance of the product during storage. An effective shipment requires good coordination and time management. Any delay in the process of logistics activity of the product, a change in the degree of heat will both financially damage the enterprise, and, more importantly, a deterioration in pharmaceutical products subject to the cold chain will adversely affect health and may even lead to fatal consequences. In order to ensure that the product does not deteriorate and is not damaged during the cold supply chain process, the pharmaceutical and medical sectors require cold chain technology more and more. In this context, this study aimed to focus on the importance of the cold chain logistics related to the transportation of Covid-19 vaccines in the pandemic days.
Logistics
A network of entities to produce and distribute services or goods from the suppliers to end-users is designated as a supply chain. Logistic is a complex process to organize and implement any operation. Logistics and supply chain management are interlinked with the plans, control mechanism, implementation, the effectiveness of forward and reverse flow processes as well as the final storage of the services and goods. There is a continuous transfer of information from point of origin to the consumers to fulfil customer requirements. The main task of distribution logistics deals with the delivery of products from the manufacturer to distributors and finally to consumers. There is some basis operational step such as processing of orders, warehousing and the transportation to the destination. These distribution logistics are very important to maintain the standards of the process and depend on the time, place, and quantity of production and their consumption parameters. Management of resources in logistics maybe some tangible goods (materials, supplies or equipment etc.), food or other consumable products [8,9].
Cold Chain Logistics
The term ‘Cold chain’ refers to a series of actions and the equipment required to maintain the quality of products in a specific low-temperature range from their production to consumption. The supply chain in cold chain setup is strictly temperature-controlled and requires uninterrupted refrigeration during productions, storage and distribution operations. There are some other activities associated with the equipment and logistics to maintain low-temperature with a specific range. The whole process is required to ensure quality, preservation and extension of shelf life of the consumer products including agricultural produce, frozen food, seafood, photographic films, pharmaceutical products and other chemicals. The transport and transient storage of such products maintaining low temperatures is sometimes termed as cool cargo. Moreover, the cold chain is considered as a science, technology and also process. It is considered as ‘science’ as the understanding of biological and chemical processes is associated with the perishability of products and goods. On the other hand, the cold chain is termed as ‘technology’ due to its reliance on physical means for ensuring desired thermal conditions. It is also a ‘process as it requires a series of tasks in manufacturing, storage, transportation and monitoring of temperature for all sensitive products [8].
Cold chains are among the common processes in the pharmaceutical, and food industries and also required for chemical shipments. The most common low-temperature range in pharmaceutical processing units is maintained between 36 to 46 °F (2 to 8 °C), but the temperature range can be specific depending on the needs of products to be shipped. Some other parameters are also considered while shipping fresh products to maintain a specific environment including air quality in terms of oxygen, carbon dioxide, humidity. These requirements complicate the operation and maintenance of cold chain processes [10]. Unlike other merchandise, cold chain goods are endangered to perishability and disability so these products always require safe and quick transportation towards end-users. These goods are transported temporarily maintaining a low-temperature environment so also termed as cold cargo during entire logistics [11].
Cold chain logistics in the transport of the Covid 19 Vaccines
Some 80 potential vaccines for Covid-19 have been launched in the market until now, but still, many research programmes are underway. To transport these vaccines from the production place to the destination, the air freight industry has to respond accordingly for efficient global transportation and delivery. Transportation and handling of Covid-19 vaccines introduced some other dimensions to supply chains. These highly sensitive and valuable vaccines require not only a temperature-controlled environment but also have to follow international regulations published by ‘EU Good Distribution Practices’, ‘US Federal Drug Administration’ and also World Health Organization (WHO) about temperature control [12].
The capacity in airfreight logistics is generated in such a way to meet all existing programmes to transport vaccines all around the world. Both the resources and infrastructure are very critical as every country was prepared to respond for massive vaccination against Covid-19 as this virus has impacted all territories to a different extent. There are some upcoming challenges for supply chain stakeholders in planning and executing delivery mechanism at the global network for Covid-19 vaccines. Collaborations are much needed in this scenario to build trust and confidence, and the integrity of sensitive vaccines is required strict maintenance throughout the process of transportation. The demand for the vaccine is increasing worldwide and to respond to this situation the ‘traditional manufacturing’ approach could be replaced with ‘distributed manufacturing’. Thus, it can be said that the decentralization approach would create multiple manufacturing units and load would be distributed among them. This would also facilitate the end-users and reduce constraints of supply chain logistics. This goal could only be met for Covid-19 vaccines if the newly developed companies ensure access to their products and compounds. Nowadays, this situation is very challenging due to the imposition of export restrictions on Covid-19 vaccines by government authorities. Such life-saving and essential medical supplies require different approvals and certification policies [13].
Covid-19 Vaccines and Smart Health Technologies in the Pandemic
The Covid-19 disease outbreak is putting a greater emphasis on protracted technological and medical advancements. Firms who are unable to provide a rich experience to their employees and clients are at risk as they traverse the new work-from-home reality. Cloud computing and cybersecurity have become key parts of organization based as companies navigate the new work-from-home reality. In the field of medicine, attempts to find a cure or better therapies are refocusing emphasis on some of the most recent advances in genetics and immunology. Significantly, innovations resulting from the imminent pandemic issue are not transient; it can be proposed entrepreneurs should broaden their horizons to see the long-term implications of current issues. Covid-19 unpredictability drives healthcare and technology innovations [7].
Vaccinations are a significant new tool to combat over Covid-19, and the fact that far too many vaccines are proven to be effective and are being developed is quite promising. Scientists from everywhere in the globe are researching and creating as swiftly as they can to deliver us diagnostics, cures, and vaccinations that would save lives and ultimately lead to the end of this pandemic [14]. There are different platforms for Covid-19 vaccines and many different vaccines developed with their various conditions (Figure 1). To speed healthcare innovation and combat the coronavirus, organizations all across the world are implementing considered trying technology as well as inventing new ones. The goal of smart healthcare is to transport data rather than people. All that is left is to hope that this future answer arrives soon, and that the promise afforded by the interplay of technological advancements is completely realized [7].
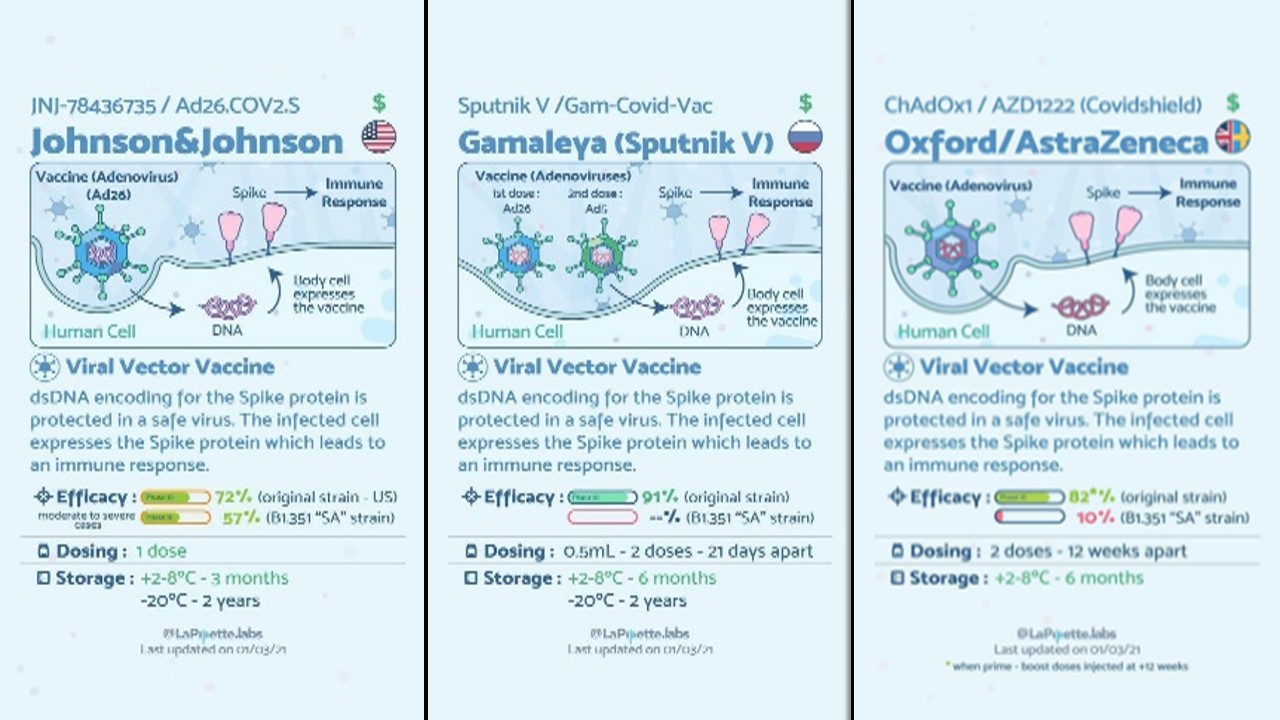
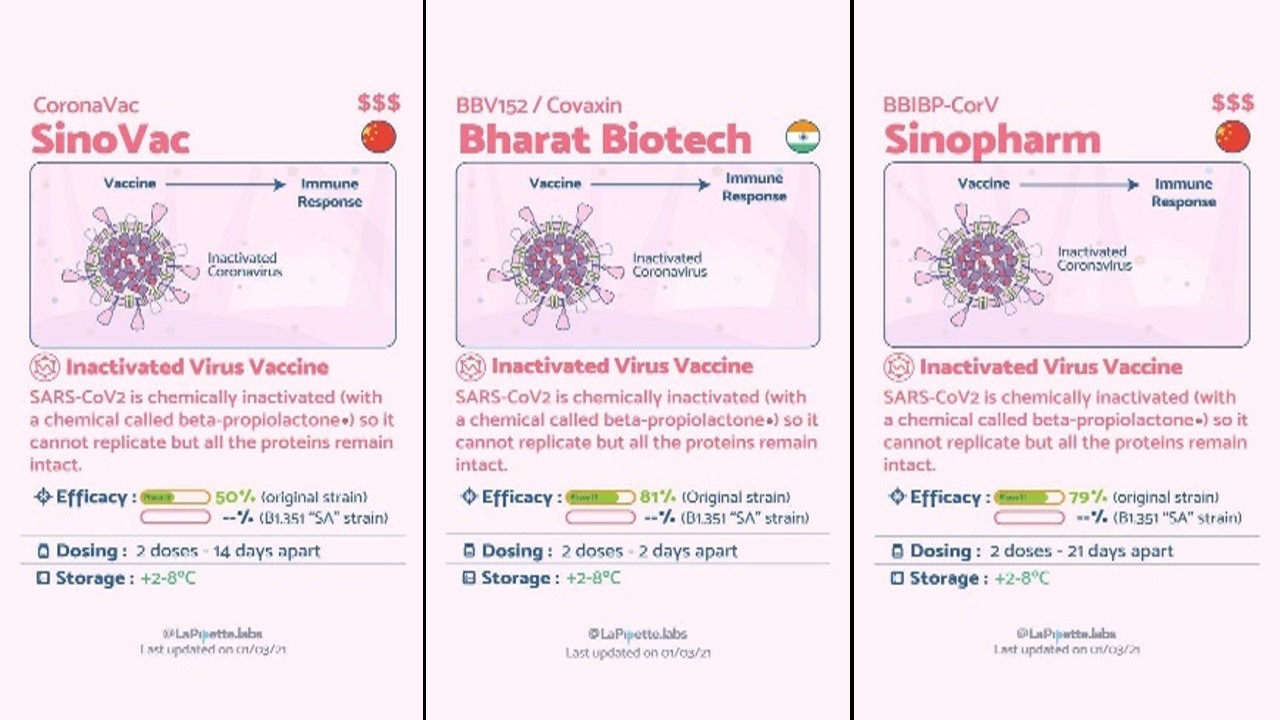
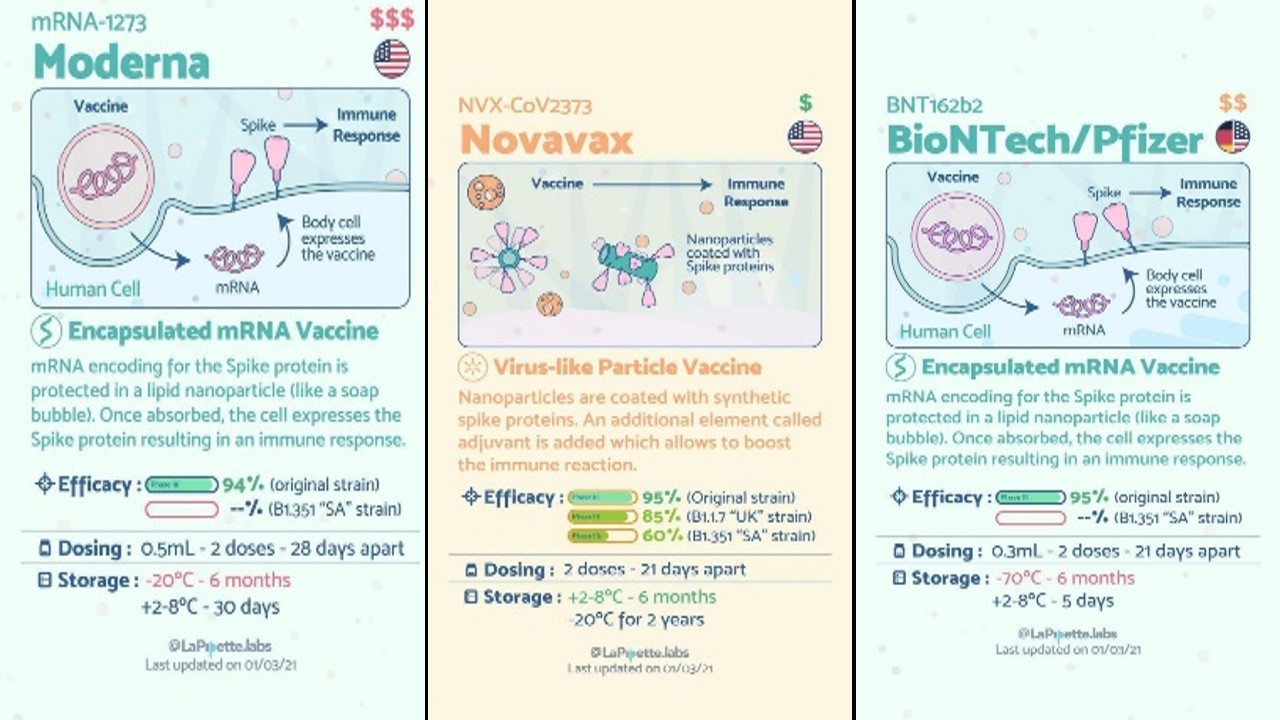
Figure 1: Different Covid-19 Vaccines with their conditions [14].
Every year, vaccines protect millions of people's lives. Vaccines function by retraining and strengthening the body's natural defenses, the immune system, to detect and combat the viruses and bacteria they are designed to combat. If the body is exposed to such disease-causing microorganisms after vaccination, the body is prepared to kill them right away, minimizing sickness. Effective and reliable vaccinations will improve the future, but for the time being, we should start wearing masks, keep a safe distance, and avoid crowds. Getting vaccinated does not imply that we may disregard prudence and put yourself and others at danger, especially because the extent to which vaccinations can defend not just against sickness but also against infection is yet unknown [14].
The temperature of most vaccinations must be kept within 1 degree Fahrenheit of their optimal temperature. Traditional immunizations are normally kept between 35- and 46-degrees Fahrenheit. Although most Covid-19 vaccinations must be maintained at temperatures below 32 degrees Fahrenheit, several of the most popular Covid-19 vaccinations must be kept at significantly lower temperatures. Pfizer's vaccine candidate needs a preservation temperature of - 94 degrees Fahrenheit, while Moderna's vaccine needs minus 4 degrees Fahrenheit. It's not simple to keep these temps consistent [14].
Once a Covid-19 vaccine is manufactured, it will mostly certainly be delivered by vehicle towards the next suitable airport. Because a Covid-19 vaccine is so important and time-sensitive, it will almost certainly be flown around across nation or over the world via air. Following the unloading of these planes, the vaccinations will be transported by truck to suitable stockroom storage facilities for distribution. Several vaccines may be shipped straight from the warehouses to the health-care institutions where they will be administered. Following the unloading of these planes, the vaccinations will be transported by truck to suitable stockroom storage facilities for distribution. Several vaccines may be shipped straight from the warehouses to the health-care institutions where they will be administered [7].
The very first stage should be to figure out where vaccinations will be manufactured. Industries might have to use vehicles and airplanes for transportation inside their own nations and for broader marketing to others if manufacturing is mostly done elsewhere. There's also some doubt regarding which Covid-19 vaccination will be licensed initially. Depending on the vaccination, different temperatures and processing protocols may be required. As a result, distinct instruction would be required for personnel across the cold chain about how to manage each vaccination [7]. Getting the right vaccines into the right people at the right time during a global pandemic is, unsurprisingly, proving to be a logistical challenge. Numerous large logistics businesses, such as UPS and DHL, are indeed making significant investments in the cold chain processing facilities. Near UPS aviation centers in Louisville, Kentucky, and Atlanta, Georgia, UPS is establishing frozen farms with 600 freezers capability of attaining - 80 degrees Celsius. The Netherlands is a country in Europe. Also every freezer would contain 48,000 vaccine bottles and so will be capable of storing whether the Pfizer or the Moderna vaccines at the required low temperatures [7]. In several localities, establishing freezers appropriate of the cold temperatures required by the Pfizer vaccine is not feasible, thus systems must be put in place to ensure that such locations receive a sufficient quantity of such vaccine. Aviation and logistical firms are assessing whether they would handle this demand. The outcome will have to wait and see. Ever other vaccination created has the potential that can save a world and gives the globe closer to routine, but delivering the vaccinations to where they are needed will be difficult. Establishing and strengthening the storage conditions for vaccine delivery would assist the globe avoid wasting vaccines and help these people overcome the pandemic quicker [7,14]. Nowadays, the globe can produce and distribute around 6.4 billion flu vaccinations each year. Analysts claim that firms will manufacture roughly 9 billion Covid-19 vaccines in 2021, and the cold storage will need to be capable of managing this massive increase on edge of the vaccines that should currently be supplied each year. According to research published in 2019, 25% of vaccinations are damaged either by time they reach their ultimate targets. When a vaccine is exposed to high temperature beyond its normal operating range and that this is discovered, the vaccinations are often discarded. A temperature error is occasionally made, and one of these vaccinations is given. According to researchers, these vaccinations have no side effects, but they may provide less immunity and need an individual to really be revaccinated. Vast majority of individuals in the United States and billions throughout the world may eventually require a coronavirus vaccination – maybe two doses. This massive immunization campaign will necessitate a complicated vaccine cold chain on a never-before-seen scale. The present vaccine cold storage is inadequate, and increasing the distribution network will be difficult [7].
So, how do businesses and government entities provide vaccinations for those that need them?
The solution is the vaccine freezing chain, which is a distribution network that can retain vaccinations at precise temperatures from the time they are manufactured until the time they are delivered to a person. A further concern is how often delivery to sites of care will be required. This will be determined by the refrigeration capacity of healthcare institutions and hospitals, personnel resources, vaccine distribution sites, and a variety of other criteria, as well as the vaccine's storage period. Finally, there is also the straightforward issue of how to increase transport and storage capability (7). Regular restaurant freezers also have range of temperatures of 5 to minus 10 degrees Fahrenheit, which is insufficient to meet the requirements needed by the Pfizer vaccine. It is necessary to use specialized equipment. It was investigated that the vulnerable product distribution networks in the pharmaceutical business and also how they relate to quality of product as just an operations management scholar. Considering trillions of vaccines required to combat the pandemic, a high spoiling rate could result in a tremendous loss of revenue as well as a significant delay in immunizations, perhaps leading to fatalities and a lengthier worldwide closure. Covid-19 vaccinations are estimated to be required in the range of 12 billion to 15 billion globally, according to experts. Vaccines are consumable products that must be stored at extremely low temperatures. The bulk of Covid-19 vaccines in production, such as those developed by Moderna and Pfizer, are RNA-based vaccines. They will spoil if they become too hot or too cold. A rotten vaccination, like damaged seafood, must be discarded. WHO is collaborating with Gavi and UNICEF to guarantee that the infrastructure as well as technical assistance are in place in order to ensure that Covid-19 vaccinations are securely given to all individuals who require them [7,14].
At minimum seven distinct vaccinations across three platforms have already been put out in nations as of February 18, 2021. Vaccination is prioritized for vulnerable groups in all nations. Simultaneously, more than 200 other vaccine candidates are being developed, with more than 60 of them in clinical trials. COVAX is a component of the ACT Accelerator, which WHO and partners introduced in 2020. COVAX, the ACT Accelerator vaccines cornerstone hosted by CEPI, Gavi, and WHO, intends to stop the Covid-19 pandemic's acute phase by; accelerating the production of safely and effectively Covid-19 vaccines; facilitating the development of production facilities; and collaborating with governments and producers to guarantee that vaccinations are distributed fairly and equally to all nations – the only global program to do so. A Covid-19 epidemic is sweeping the globe. WHO and collaborators are scrambling to develop and implement safely and effectively vaccinations while they work together over the reaction — monitoring the epidemic, consulting on essential measures, and sending important medical supplies to individuals in need [7].
Pharmaceutical and biochemical engineering items must be handled and transported under strict guidelines, otherwise the drug may lose its efficacy and become useless. This adds to the present constraints of restricted aviation freight capacity and worldwide connection as a result of the cancellation of roughly two-thirds of the commercial network. Handling and delivering vaccinations adds a new layer to the logistics of the supply chain - it's not simply a box! Such elevated and delicate items may necessitate not just a temperature-controlled development environment, but also must adhere to international regulatory criteria outlined in the Temperature Control Regulations [14].
The majority of temperature errors in the cold storage facilities are caused by ineffective transportation practices, with yearly losses approximated at $34.1 billion. However, that figure does not include the cost – both financially and physically – of any sickness that could've been avoided if high-quality vaccinations had been delivered on time. Aircraft, trucks, and cold storage facilities are all necessary components of the cold chain. The vaccine manufacturing sites and demand points determine how and why the infrastructure is interconnected and exploited [7]. Todays modern air cargo logistical capacity is geared to match each country's present scheduled immunization programs. As governments prepare for a huge vaccine reaction to Covid-19 that will affect all nations and territories, including infrastructure and manpower will be important. The supplier stakeholders' next task is to create and implement a worldwide network distribution strategy for the Covid-19 vaccines, which has never been done before. Would this temperature-controlled distribution network be able to handle, store, and transfer such a significant increase in vaccine quantities? -Certain transporters, ground handlers, forwarders, and transporters may be confused how to manage temperature-sensitive items successfully. Furthermore, because temperature-controlled life science medical supplies may not have been acceptable for travel in the passenger cabin, pharmaceutical producers may be less reluctant to have their precious goods handled in this fashion. As a result, before accepting or handling vaccine shipments, all suppliers must acquaint themselves with either the overall criteria for securely processing vaccine deliveries. It may be necessary to dedicate special or supplementary resources inside their networks, as well as assign additional and/or legally compliant vaccine storage capacity. It's possible that industry retraining and compliance certifications may have to be expanded [7,14].
Conclusion and Suggestions
The vaccine manufacturing industries are largely influenced by the contribution from intermediate industries in the locality end user’s destinations. The major portion of vaccines are imported from foreign countries, the bottleneck strategy may prevent pharmaceutical industries from maximizing their impact along the supply chains. In such a situation, domestic production could be boosted if the policymakers execute supportive policies which would, in turn, reduce the import of vaccines. This would also facilitate the pharmaceutical industry to flourish and its production would also increase manifolds. Expectedly, the producers of vaccines and vaccine services are the two most important industries which have globally influenced vaccine processing as they are major users of the supply chain during a pandemic. Cold chain logistics is the life of pharmaceutical products such as the Covid-19 vaccine products in the pandemic.
The continuous supply of vaccines could only be ensured by cooperation, collaboration and communication. Prompt actions are needed to initiate industrial transformation that could only be achieved through an efficient, integrated, and collaborative supply chain mechanism. Whether in alignment with globally harmonized standards or with the industrial collaborative efforts, by digitization, data sharing, risk assessment, or by tracking and tracing strategy. This improved approach will lead to growing expectations, transparency and high standardization across supply chains. The airfreight logistics is ambitious and must follow some workable plans for resilient solutions.
There has been a collaboration between logistics and pharmaceutical industries for many decades to improve the quality of products within realistic capabilities. The logistics in general and cold chain logistics, in particular, will continue their vital contribution in supplying life-saving products to help fight against the coronavirus and to diminish its impacts on humanity. It is highly believed that laboratory scientists with support from medical professionals, industries, and public health authorities will eventually overcome this pandemic.
More information regarding this Article visit: OAJBGSR
https://biogenericpublishers.com/pdf/JBGSR.MS.ID.00241.pdf
https://biogenericpublishers.com/jbgsr-ms-id-00241-text/

No comments:
Post a Comment