Mir-146a Inhibits IFN-Γ Production Via Suppressing TLR4/IRAK-1/NF-kb Expresion in Pulmonary Arterial Smooth Muscle Cells by Shuyuan Chu in Open Access Journal of Biogeneric Science and Research
Abstract
Purpose: The microRNA-146a (miR-146a) could regulate proliferation of vascular smooth muscle cell and inhibits inflammation of airway, but its role in inflammation of pulmonary arterial smooth muscle cell (PASMC) hasn’t been reported. We aim to explore the effect of miR-146a on regulating inflammatory signaling in the study.
Methods: Primary PASMCs were separated from rats. Cells were stimulated by lipopolysaccharides (LPS). miR-146a was transfected into cells with plasmid. miR-146a expression in PASMCs was assessed by real-time PCR. The protein expression of TLR4, phosphorylated-IRAK-1, phosphorylated-IKK, phosphorylated-IκB and NF-κB (P65) in PASMCs was analyzed using western blotting. The level of IFN-γ was detected using ELISA.
Results: The protein expression of TLR4, phosphorylated-IRAK-1, phosphorylated-IKK, phosphorylated-IκB and NF-κB (P65) in PASMCs was increased when induced by LPS, which was reversed by miR-146a. The level of IFN-γ in supernatant of PASMCs was higher in LPS-treated group than controls, which was decreased in cells with miR-146a overexpressed.
Conclusion: miR-146a could attenuate LPS-induced IFN-γ production, and activation of TLR4, IRAK-1 and NF-κB in PASMCs, which might provide novel target on the therapy of pulmonary hypertension.
Introduction
Pulmonary hypertension (PH) is a hemodynamic and pathophysiologic syndrome from increased blood pressure within pulmonary arteries, which prevalence is approximately 10 % in general population. Its prognosis is depressed that the one-year mortality is approximately only 15% [1]. Pulmonary arterial smooth muscle cell (PASMC) participates in PH through activating inflammatory signaling, such as NF-κB pathway [2,3]. However, the precise mechanisms of inflammation in PASMC are not very clear.
Recent studies have showed that microRNA-146a (miR-146a) could regulate proliferation of vascular smooth muscle cell from aortic artery [4,5] In addition, miR-146a could reduce inflammation in airway by targeting on IRAK-1 [6]. However, the role of miR-146a in inflammation of PASMC hasn’t been reported. Interestingly, miR-146a was found to contribute to inhibiting lipopolysaccharides (LPS)-induced activation of TLR4/IRAK1/NF-κB signaling in monocytes [7]. Similar finding was showed in intestine epithelial cells [8]. These findings suggested that miR-146a could inhibit the activation of TLR4/IRAK1/NF-κB signaling in inflammation. Moreover, our previous work found that LPS could induce the activation of TLR4/IRAK-1/NF-κB signaling, resulting in an increased production of IFN-γ in PASMCs [9]. Thus, in this study, we explore the role of miR-146a in regulating IFN-γ production and TLR4/IRAK-1/NF-κB signaling activation in PASMCs.
Methods
Cell Culture and Transfection
Male Wistar rats (8-10 weeks old, weighing 280±20 g) were obtain from experimental animal center of Guilin Medical University. All experimental procedures were approved by the Animal Care and Use Committee of the Affiliated Hospital of Guilin Medical University. Rats were anaesthetized with 5% isoflurane by inhalation in oxygen and killed by cervical dislocation. The small vascular was separated from the 3rd level or lower artery branch of pulmonary lobe segments, and then was minced to small pieces and digested by 0.2% type I collagenase for 20 min at 37 °C in water. Digestion was stopped by adding 10% FBS (GIBCO, MA, USA)). The primary PASMCs were cultured in DMEM medium containing 10% FBS at 37°C in 5% CO2. Seven days later, PASMCs at passages 3-6 were used to conduct the experiments. Cells were cultured in serum-free medium 30min prior to transfection.
The primary PASMCs were identified using immunohistochemistry with α-SM-actin staining (Figure 1). The slides of cells were fixed by 4% paraformaldehyde for 20 min and incubated in 0.6% H2O2 for 30 min to quench endogenous peroxidase activity. The slides were incubated with primary mouse anti-rat antibody against α-SM-actin (dilution 1:100, BM0002, BOSTER, Wuhan, China) at 4 ◦C overnight, and then were incubated with horseradish peroxidase conjugated goat anti-mouse IgG antibody (BA1001, BOSTER, Wuhan, China) at room temperature for 20 min. After washes with PBS for three times, 3’3-diaminobenzidine-tetrahydrochloride was applied on the slides as a chromogen for 1–5 min, and were then by haematoxylin for 5–10 min. The transfection of miR-146a was performed with plasmid (Genechem, Shanghai, China) and lipofectamine2000 (Invitrogen, MA, US) according to the manufacturer’s instruction. When six hours after transfection, LPS-induced cells were stimulated with LPS(1μg/ml) (Sigma, MO, US) for 48 hours.
Quantitative Real-Time PCR
PASMCs (1×106cells/well) were plated into six-well plates and incubated overnight in a humidified incubator at 37 ◦C in 5% CO2. Total RNA was extracted using the RNA simple Total RNA Kit according to the manufacturer’s protocol, and RNA was reverse transcribed into cDNA. A quantitative real-time polymerase chain reaction (PCR) was performed using Hairpin-itTM microRNA and U6 snRNA Normalization real time-PCR quantitation kit (GenePharma, Shanghai, China)) with ABI PRISM 7500 Sequense Detection System (Thermo Fisher Scientific, Inc, Carlsbad, California, USA) in accordance with the manufacturer's protocol. The 20μl PCR reactions (with 10μl Real-time PCR Master Mix, 0.4μl microRNA-146a /U6 snRNA specific Primer set, 0.2μl microRNA-146a /U6 snRNA specific Probe, 0.4μl ROX reference dye, 0.2μl Taq DNA polymerase, 2μl miRNA RT product and 6.8μl PCR H2O) were undergone 3 min at 95 ◦C, then 40 cycles of 12 s at 95 ◦C and 40 s at 62 ◦C. RT and PCR primer sequences are as follows: miR-146a RT: GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAACCCAT, miR-146a FP: GGCGTGAGAACTGAATTCCA, miR-146a RP: TCGTGGAGTCGGCAATTG; U6 RT: CGCTTCACGAATTTGCGTGTCAT, U6 FP: GCTTCGGCAGCACATATACTAAAAT, U6 RP: CGCTTCACGAA TTTGCGTGTCAT. The level of mRNA expression was reported as fold change using the 2–△△CT method. Every sample was triplicated.
Western Blot Analysis
PSMCs were treated with 100μl RIPA and PMSF (100:1) for 30 min on ice, and then centrifuged at 12000 × g (4 ◦C) for 20 min. The loaded proteins (15μg) were separated on a 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transferring onto PVDF membranes. After blocking with 5% non-fat dried milk for 2 h at room temperature, the membranes were incubated with mouse anti-rat antibodies against TLR4 (dilution 1:500; Abcam, MA, USA)), IKK (dilution 1:5000; Abcam, MA, USA), β-actin (dilution 1:1000; ZSGB-Bio, Beijing, China), rabbit anti-rat antibodies against IRAK-1 (dilution 1:500; Abcam, MA, USA), IκB (dilution 1:1000; Abcam, MA, USA), and NF-κB (p65) (dilution 1:1000; CST, MA, USA) overnight at 4 ◦C, and then were incubated with horseradish peroxidase conjugated goat anti-mouse or anti-rabbit(dilution 1:5000) for 1 h. Finally, the blots were developed with the ECL Plus reagents (Bio-Rad, USA).
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay (ELISA) was used to detect the level of IFN-γ in cell culture supernatants according to the protocol of ELISA kit (Elabscience, Wuhan, China). Each sample was repeated in three wells. Briefly, in 96-well plates, 100μl sample and 100μl biotinylated detecting antibody (50μl cells and 50μl Detection reagent A) were incubated for 1 h at 37 °C, followed by incubation with 100μl Horseradish-peroxidase (HRP) conjugated working solution for 30 min at 37 °C. Subsequently, plates were incubated with substrate solution as a chromogen for 15 min without light. The optical density (OD) was measured at 450 nm using a microplate reader (TECAN, Switzerland).
Statistical Analysis
All statistical analyses were performed using SPSS 21.0 (IBM SPSS Inc., Chicago, IL, USA). Group data are expressed as mean ± std. deviation (SD). Significant differences were evaluated using an independent-samples t-test, and multiple groups were compared using one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test or the Games–Howell test. p-values < 0.05were considered to be statistically significant.
Results
miR-146a Inhibits TLR4 Expression in PASMCs
When PASMCs were transfected with miR-146a, the expression of miR-146a was respectively increased about 6-fold at the 24th hour and 18-fold and at the 48th hour (Figure 1A). This demonstrated the successful transfection of miR-146a. Moreover, the expression of miR-146a was significantly induced by LPS after 24-hour administration (Figure 1B). That effect was time-dose dependent. Furthermore, the protein expression of TLR4 in PASMCs was detected after miR-146a transfection. TLR4 expression was increased in LPS group compared with controls, whereas it was reversed when transfected with miR-146a (Figure 2). Thus, miR-146a could inhibit TLR4 expression in PASMCs.
miR-146a Inhibits IRAK-1 Activation in PASMCs
The activation of IRAK-1 in PASMCs was detected by Western blotting. The protein expression of phosphorylated-IRAK-1 (Figure 3) was increased when treated with LPS. However, it’s reduced in cells with miRA-146a overexpression. These findings suggest that miR-146a could inhibit IRAK-1 activation in PASMCs.
miR-146a inhibits IKK activation in PASMCs
The activation of IKK, IκB and NF-κB (P65) in PASMCs was detected by western blotting. The protein expression of phosphorylated-IKK (Figure 4), phosphorylated-IκB (Figure 5) and NF-κB (P65) (Figure 6) was increased when treated with LPS. However, it’s reduced in cells with miRA-146a overexpression. These findings suggest that miR-146a could inhibit IKK activation in PASMCs.
miR-146a inhibits the secretion of IFN-γ in PASMCs
The level of IFN-γ in the supernatant of PASMCs culture medium was assessed by ELISA. (Figure 7) illustrates that the level of IFN-γ was higher in LPS group than controls. In contrast, it’s decreased in cells with miRA-146a overexpression. These findings indicate that miR-146 could inhibit IFN-γ secretion in PASMCs.

Figure 1: miR-146a expression in PASMCs. (A) miR-146a is overexpressed in PASMCs when transfected with Rno-mir-146a plasmids. The expression level of miR-146a was respectively increased about 6-fold at the 24th hour and 18-fold and at the 48th hour. (B) miR-146a expression is induced by LPS. The expression of miR-146a was significantly increased after 24 hours induced by LPS. That effect was time-dose dependent. *: p<0.05, **: p<0.01

Figure 2: miR-146a inhibits TLR4 expression in PASMCs. PASMCs were transfected miR-146a expressing plasmids. TLR4 expression in PASMCs was increased in LPS group, whereas it was reversed when transfected with miR-146a. The representative images are shown in left panel and quantitative analysis results are shown in right panel. **: p<0.01.

Figure 3: miR-146a inhibits IRAK-1 activation in PASMCs. PASMCs were transfected miR-146a expressing plasmids. Phosphorylated-IRAK-1 expression in PASMCs was increased in LPS group, whereas it was reversed when transfected with miR-146a. The representative images are shown in left panel and quantitative analysis results are shown in right panel. * p<0.05, ** p<0.01.

Figure 4: miR-146a inhibits IKK activation in PASMCs. PASMCs were transfected miR-146a expressing plasmids. Phosphorylated-IKK expression in PASMCs was increased in LPS group, whereas it was reversed when transfected with miR-146a. The representative images are shown in left panel and quantitative analysis results are shown in right panel. * p<0.05, ** p<0.01.

Figure 5: miR-146a inhibits IκB phosphorylation in PASMCs. PASMCs were transfected miR-146a expressing plasmids. Phosphorylated-IκB expression in PASMCs was increased in LPS group, whereas it was reversed when transfected with miR-146a. The representative images are shown in left panel and quantitative analysis results are shown in right panel. **: p<0.01.
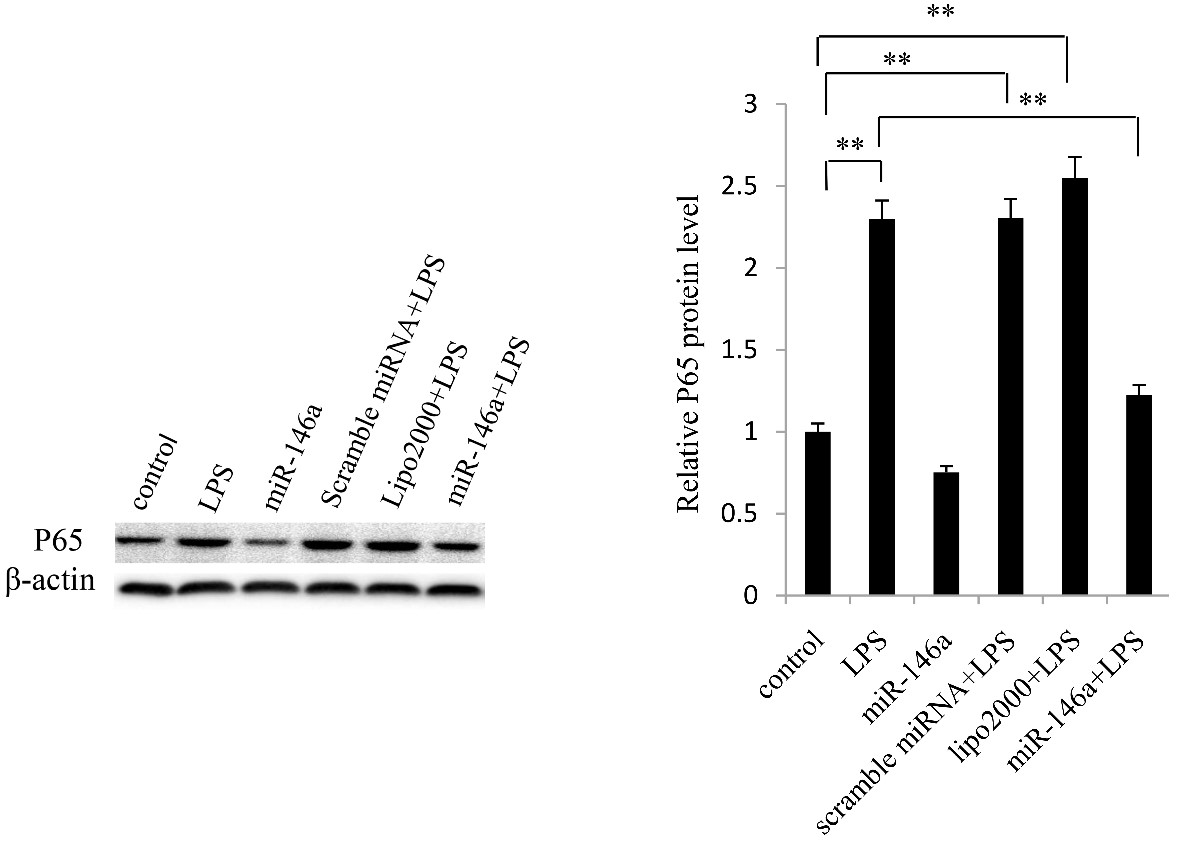
Figure 6: miR-146a inhibits NF-κB (p65) expression in PASMCs. PASMCs were transfected miR-146a expressing plasmids.NF-κB (p65) expression in PASMCs was increased in LPS group, whereas it was reversed when transfected with miR-146a.The representative images are shown in left panel and quantitative analysis results are shown in right panel. **: p<0.01.

Figure 7: miR-146a inhibits the secretion of IFN-γ in PASMCs. PASMCs were transfected miR-146a expressing plasmids. Six hours after transfection, the cells were treated with LPS(1μg/ml) until 48 hours post-transfection. The cell culture medium supernatant was collected and IFN-γ production was measured by ELISA. The level of IFN-γ was increased when treated with LPS, which could be reversed in cells with miR-146a overexpression. *: P<0.05, **: P<0.01.
Discussions
Our study shows that miR-146a could attenuate LPS-induced IFN-γ production, TLR4 expression, and activation of IRAK-1 and NF-κB in PASMCs. The present study confirmed our previous finding that LPS could induce IFN-γ production, [9] and further found that miR-146a could significantly inhibit LPS-induced IFN-γ production. In vascular smooth muscle cells, IFN-γ could stimulate NF-κB activation, leading to inflammation [10] Those findings suggest that in vascular smooth muscle cells, IFN-γ may be not only an effector of LPS stimulation, but also a stimulator in the process of inflammation. It may play a key role in positive feedback of inflammation. Thus, it’s meaningful to disturb that feedback for reducing inflammation in PH treatment. The miR-146a may be a potential target since it could reduce LPS-induced IFN-γ production in PASMCs as it’s found in our study.
TLR4 is a crucial signaling in promoting inflammation of vascular smooth muscle cells [11-14]. Our study found that TLR4 expression in PASMCs was increased in LPS group, whereas it was reversed when transfected with miR-146a. The miR-146a could regulate TLRs and downstream signaling through TNF receptor-associated factor 6 and IL-1 receptor-associated kinase [15]. Thus, our findings suggest that miR-146a could suppress LPS-induced TLR4 expression in PASMCs.
Furthermore, TLR4 could activate NF-κB singling in LPS-induced inflammation of vascular smooth muscle cells from thoracic aortas [16,17]. Similarly, IRAK-1 is also the downstream of TLR4 in vascular smooth muscle cells from thoracic aortas [18]. In pulmonary vascular smooth muscle cells, TLR4 could activate IRAK-1/NF-κB signaling.9 Therefore, we explore the role of miR-146a in the activation of TLR4/IRAK-1/NF-κB signaling in PASMCs in the present study. This study showed that when miR-146a was overexpressed, the LPS-induced activation of IRAK-1 and NF-κB singling in PASMCs was inhibited. Since TLR4/IRAK-1/NF-κB singling in PASMCs could be activated by LPS and then lead to IFN-γ production, [9] we supposed that miR-146a could attenuate the activation of TLR4/IRAK-1/NF-κB singling, resulting in the decreased production of IFN-γ. Therefore, miR-146a may be a potential therapeutic target on inflammation of pulmonary artery, which may provide novel avenues in the therapy of PH.
Conclusion
In conclusion, miR-146a could attenuate LPS-induced IFN-γ production via inhibitingTLR4/IRAK-1/NF-κB pathway in pulmonary arterial smooth muscle cells, which might provide novel target on the therapy of PH.
More information regarding this Article visit: OAJBGSR

No comments:
Post a Comment