Antibacterial Behaviour of Graphene Oxide Nanoplatelets (GO)- Polyvinyl Alcohol (PVA)- Polyvinylpyrrolidone (PVP) Biopolymer Composite Films by Biswadeep Chaudhuri* in Open Access Journal of Biogeneric Science and Research
Abstract
We have prepared a higher conducting 80wt% polyvinyl alcohol (PVA)-20wt% polyvinyl pyrolidone (PVP) blend. Addition of 0.05wt% graphene oxide nanoplatelets (GO) in this blend further enhanced the conductivity of the composite blend. Antibacterial characteristics of this graphene oxide embedded 80wt%PVA-20wt%PVP composite films towards the bacterial strains: Gram-positive Lactobacillus sporogens, Clostridium butyricum, Bacillus mesentricus and Streptococcus faecalis have been investigated showing improved antibacterial properties of the composite films.
Keywords: Polymer composite, Biomaterial, Antibacterial, Graphene oxide
Highlights
- Synthesized Graphene oxide nanoplatelets (GO)-PVA-PVP composite films.
- GO addition enhanced conductivity of thePVA-PVP biopolymer films
- (GO)- PVA-PVP composite films showed antimicrobial properties
Introduction
Polymer nano-composites have drawn special interest for their electronic and biomedical applications [1,2]. Because of extraordinary physicochemical properties, graphene oxide (GO) and reduced graphine oxide (rGO) are considered as important filler materials for composite materials. Recently GO embedded bio polymers like polycaprolactone (PCL), poly-lactic-co-glycolic acid (PLGA), Polyvinyl alcohol PVA and poly lactic acid (PLA) have shown superior biocompatibility suitable for tissue engineering and other biomedical applications [3-6].Graphene and GO have been proved to exhibit excellent biocompatibility and high antibacterial activity [7]. Graphene oxide based polymer composites showed enhanced conductivity which supported better cell scaffold interaction and biocompatibility [8]. It appears that during direct contact with the bacteria, sharp edges of GO nano sheets induce membrane stress by puncturing or penetrating into the cell membranes, thus leading to morphological destruction of bacterial cells and leakage of intracellular components, such as proteins, phospholipids, RNA, and DNA. It was reported that with the addition of PVP to the host PVA (both water soluble insulating polymers) conductivity of the blend increased. The 80wt% PVA-20wt% PVP blend (PVPA) showed highest conductivity enhancement in the blend. The PVA-PVP blend is important for using as electrode material [9]. This blend was also reported to be suitable for wound dressing [10]. Though biocompatibility of many biopolymer GO composites have been investigated [3,5,8,11] antibacterial properties of GO embedded biocompatible polymer composites have so far been barely studied.
In the present study, we have investigated the antibacterial characteristics of 0.05wt%GO-PVPA composite (referred to as GOPVPA) films towards different bacterial strains Lactobacillus sporogens, Clostridium butyricum, Bacillus mesentricusand Streptococcus faecalis. Several other studies like Agar-diffusion test, Microbial-viability test, Swarming Motility assay, Static assay and Fimbraie production assay were also made supportin the investigation.
Materials and Method
Polyvinylpyrrolidone (Mw: 40000) and PVA (Mw: 131000) were procured from Loba Chemical, India. The graphene oxide platelets were prepared by the oxidation of graphite and the exfoliation of the generated graphite oxide via Hummer’s method [12]. X-ray diffraction (XRD) study was carried out using a PHILIPS SHIFFERT 3710 diffractometer supported by Lynx Eye super-speed detector and Ni-filtered Cu-Kα (λ=0.15406 nm) radiation generated at 40 kV/ 40 mA. The surface morphologies of CFP-PVA composite films were studied by means of scanning electron microscope (SEM: JEOL Model JSM-6490) with an accelerating voltage of 15 KV. FTIR spectroscopic studies of PVA, CFP and CFP-PVA composite were carried out in the frequency range from 4000 to 400 cm−1 using the Perkin Elmer Spectrum Version (model spectrum 2 series, NIOS2 Main software, USA) interfaced with personal computer (PC) for data processing. FTIR spectra were investigated at ambient temperature, obtained by performing four scans at a resolution of 4 cm-1. Conductivity of the composite films was measured similarly to our previous report [13].
Preparation of Gopvpa Films
To prepare GOPVPA composite (with GO concentrations y=0.05 wt% ), requisite amount of GO was dissolved in water and treated with ultrasound for 45-50 minutes to make a homogeneous brown dispersion. 80%PVA-20wt%PVP (PVPA) was first dissolved in distilled water at 90 and the solution was subsequently cooled to room temperature (RT). The GO aqueous solution was then gradually added to the PVPA solution with stirring and sonicated at RT for 30 minutes to obtaining homogeneous GOPVPA solutions. Finally, the above solutions were allowed to stand overnight to move the air bubbles and then poured into Teflon dishes and kept at 40oC for film formation until its weight equilibrated. The smooth, uniform thin films of different thickness (0.05-0.1mm) with good mechanical properties were obtained.
Cultivation of Bacterial Strain: The strains were isolated from Bifilac and grown in Luria Bertani (LB) broth and grown statistically for 24-48hr.
Agar-Well Diffusion Test
The antimicrobial activity of the GOPVPA was tested by placing 0.5mm of GOPVPA film over lawn culture of different bacterial strains. It was incubated at 37oC for 24 hrs and then studied for zone of inhibition.
Microbial-Viability Test
All bacterial strains were precultured in LB broth and then bacterial cells were diluted with PBS to 1 x 105 CFU/mL and 0.1mL is dispensed on the GO Film whereas Bacterial cells dispensed on an empty well served as a control. To avoid vaporization of bacterial cell, a wet condition is maintained by placing the 60mm Petri dish into 100mm petri dish containing wet tissue layer. About 0.02ml of harvested bacterial suspension, harvested after 4hr incubation at 37◦C, was then mixed with 0.980ml of PBS. The diluted 1ml bacterial suspension was then lawn cultured over Luria Bertani Agar plate and incubated at 37◦C for 48hrs and then colonies was calculated.
Loss of viability (%) = (counts of control − counts of samples incubated with 2D sheet surfaces)/counts of control × 100 (%) …………. (1)
Swarming Motility Assay
For checking swarming motility of bacteria, 0.01mL of overnight culture (1x105 CFU/mL) of the bacterial strain was spotted on the centre of LB agar (0.5 %) supplemented with glucose (0.8%)and incubated at 37◦C. To check the effect of film over swarming motility of bacteria, 0.5mm size films were placed at the centre of plate and 0.01mL of culture was placed over it and swarming pattern was observed for 24, 48, 72hrs and so on.
Biofilm Static Assay
To determine the amount of biofilm produced by bacteria and the effect of GOPVPA film over it, we performed invitro biofilm static assay using crystal violet dye. GOPVPA film (cut into 0.5mm size) was then placed in test tube containing LB broth and then autoclaved. Then 1mL of 1x105 CFU/mL bacterial colonies was added alone as a control variant and to each autoclaved test tube with the films and kept for incubation at 37◦C for 8hr. After incubation, test tubes were taken out and media along with film was dumped. Test tube washed twice with Millipore water to remove unattached cells and then left to dry. Then 2mL of 0.1% crystal violet was added in each test tube and incubate for 15min. Test tubes were rinsed twice with Millipore water to remove excess stain and then allowed to dry for 1hr. At end, 3 ml of 80% ethanol was added to each test tube and allowed to stand for 15min till dye dissolved completely in the ethanol. It was then analyzed by spectrophotometer at OD595 which was directly proportional to the amount of biofilm produced bybacteria.
Fimbriae Production Assay
To evaluate the morphology of Fimbriae in Lactobacillus sporogens, Bacillus mesentricusand Streptococcus faecalis, about 0.01mL of bacterial culture (1x105 CFU/mL) was spotted on the centre of LB agar (15g/L) supplemented with congo red (20µg/mL) and coomassie brilliant blue (10µg/mL). To measure the effect of film over fimbriae production, films were placed at the center of plate and bacterial suspension was spotted over it and production was measured after 24-72hr of incubation at37◦C. The attachment and the ability to form biofilm over the surface of the films were observed by FESEM. The incubated film with bacterial strain was first washed with PBS and then fixed with 2.5% glutaraldehyde and subsequently dried by graded series of ethanol (10,20,30...100%) and was then coated with osmium and 3Kv electron beam with magnification of 104.

Figure 1: (a) x-ray diffraction pattern (a), (b) FTIR (b) and (c) Raman spectra and (d) Electrical conductivity of GOPVPA film.
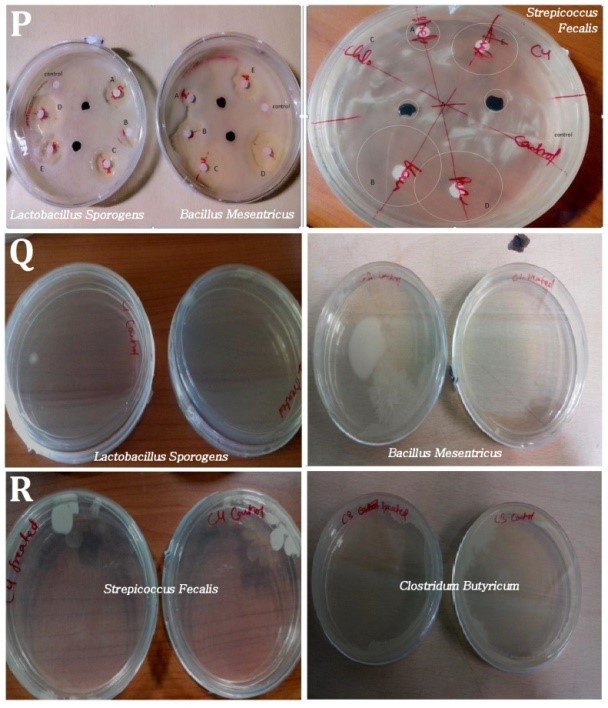
Figure 2: (P) Agar diffusion test of bacterial strain and GOPVPA composite films (Different antibiotic disk were used for comparison. A: Streptomycin, B: Kanamycin, C: Chlororamphenicol, D: Penicillin, E: Ampicillin). (Q,R) After incubation of strain with film, they were lawn cultured and observed for the growth of colonies,

Figure 3: Bar diagram indicates quantified data of Biofilm production assay. Blue colour bar represents biofilm production in absence GOPVA. Red showed production in presence of film. The decrease in production was more in case of Lactobacillus sporogens and Clostridium butyricum. Iit was less in case of Streptococcus faecalis.
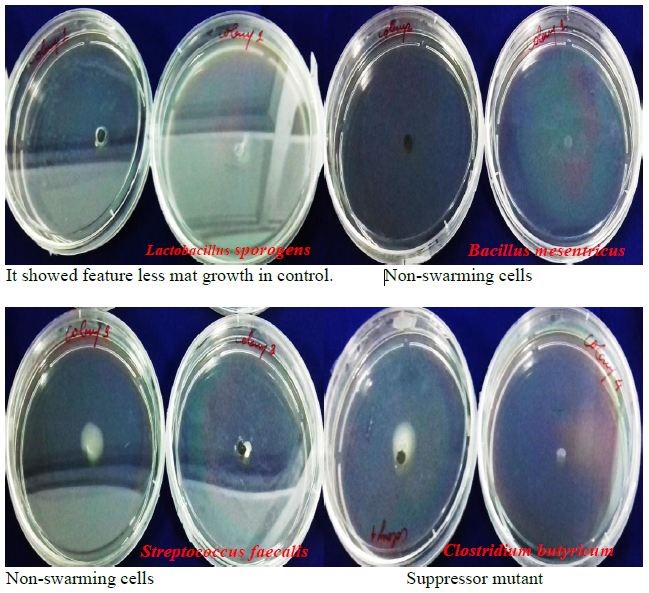
Figure 4: Swarming motility assay done to analyze the swarming pattern of different bacterial stain in presence and absence of GOPVPA film.
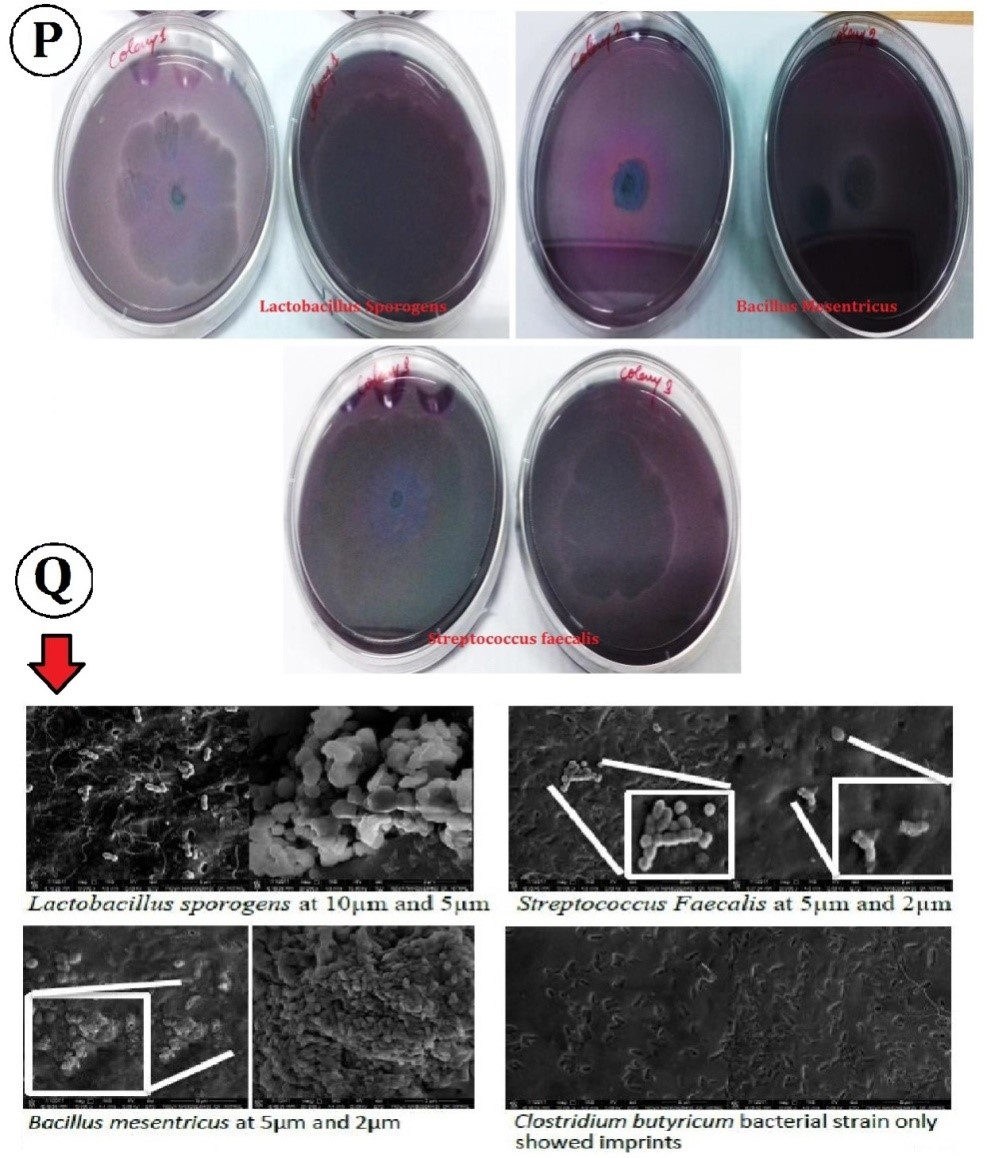
Figure 5: (P) Fimbraie production analysis by colony morphology of bacterial strain in presence and in absence of GOPVPA film, (Q) FESEM analysis of bacterial strain morphology change in presence of GOPVPA film.
Table 1: Loss of viability.

Results and Discussion
(Figure 1(a)) showed the XRD of GOPVPA indicating the characteristic GO peak (101). Characteristic FTIR spectra of the composite reported in (Figure 1b) showed small absorption band around 1539 cm-1 which could be assigned to the characteristic vibration of C=N (pyridine ring) [14]. absorption band at 962 cm-1 is assigned to the out-of-plane rings C-H bending. The wide absorption band at about 3385cm-1 is attributed to O-H stretching vibration of hydroxyl group in PVA. The band corresponding to CH2 asymmetric stretching vibration appeared around 2939 cm-1in PVP [15]. Raman spectra of GOPVPA (Figure 1c) indicated the characteristic GO peaks at frequencies around 1349 and 1604cm-1, respectively, for the G and D band usually assigned to the E2g phonon of Csp2 atoms and a phonon breathing mode of symmetry A1g.. Observed conductivity of the GOPVAP film is higher than that of PVA-PVP blend film by more than 3 orders of magnitude (Figure1d). Conductivity enhancement is important for cell growth and proliferation [8].
Agar Diffusion Test
The 24h of incubation did not show any growth over the film, indicating that it might have antimicrobial property. However, a slight zone was observed around the disk initially with Streptococcusfaecalis (Figure 2P)
Microbial-Viability Test
Viability of the bacterial strain decreased when bacterial strains were made to come in contact with the GOPVPA film directly. However, effect was maximum in Bacillus mesentricusstrain. Loss in viability (LV) was calculated by calculating the number of colonies per plate in both control and treated one (Figures 2Q,R). The loss of viability (%) was shown in (Table 1).
Biofilm Static Assay
Biofilm static assay was done to enumerate the decrease in bacterial population due to killing effect of GOPVPA biofilm. There was a marked difference in the production of biofilm by bacterial strains in presence and in absence of GOPVPA film. Maximum inhibition was seen in lactobacillus and clostridium butyricum strains. The enumerated data was concised in graphical format and shown in (Figure 3).
Motility Assay
The swarming ability of bacterial strain was assessed in presence of GOPVPA film, when they came in contact with the film in all cases, the swarming rate got decreased. Bacterial strains showed different swarming pattern (Figure 4).
Production Assay
The morphology of the fimbriae produce by bacterial strain in presence of GOPVPA film was different to that produced in absence of it. The amount of fimbriae produced was more in absence of film to that in its presence. Bacterial strain showed ruguose growth in plate after 48hr of incubation. Fimbraie production was analysed for bacterial stains, Lactobacillus sporogens, Bacillus mesentricusand Streptococcus faecalis (Figure 5P). The ability of bacterial strain to form biofilm and its colony morphology was analyzed by (Figure 5Q). Distortion in the shape of bacteria was observed under high magnification. It also showed that clostridium butyricum left imprints over the film indicating its inability of attachment with film.
Conclusion
We have synthesized a higher conducting 80wt%PVA-20wt%PVP polymer blend embedded with (0.05wt%) graphene oxide nanoplatelets (GO) and investigated the antibacterial activity of the composite film with different bacteria. Addition of GO enhanced conductivity of the pure 80wt%PVA-20wt%PVP polymer blend and the GO-80wt%PVA-20wt%PVP (GOPVAPA) composite showed antibacterial property against different bacterial strains. The multifunctional biopolymer composite GOPVPA would be suitable for biomedical and other electronic applications. Further research n this direction is in progress.
More information regarding this Article visit: OAJBGSR

No comments:
Post a Comment