A Case-Study of the Physico-Chemical Parameters of the Public Water Supply in the University of Port Harcourt by Johnson Ajinwo OR in Open Access Journal of Biogeneric Science and Research
Abstract
Water –borne diseases is on the rise currently in the third world countries as a result of lack of routine water analysis checks to ensure that the desired quality of drinking water is upheld. In the light of the above, this research aimed at determining the physico-chemical properties and mineral content of seventeen water samples from the students’ residential areas and environs of the Main Campus of the University of Port Harcourt, Choba, Rivers State, Nigeria was carried out. The results showed that most of the physico-chemical quality indices of the water samples were within acceptable limits, except the nitrate levels of samples 13 and 14. The pH of all the samples were found to be acidic, with sample 12 having the lowest pH of 4.44. The hardness levels of the samples were determined to be very soft affirming the relationship between acidic pH and soft water. This increase in the corrosivity and plumbosolvency of the samples may result in long-term risk of metal poisoning from plumbing materials. However, the metal analysis showed only slight sodium and calcium contamination which may pose no health risk.
Introduction
About 829,000 people die annually from diarrhoea caused by poor sanitation, hand hygiene and drinking contaminated water. A number of diseases which include cholera, dysentery, diarrhoea, polio, typhoid and hepatitis A are transmitted through contaminated water and poor hygiene. Deaths from contaminated water are preventable and efforts aimed at tackling this ugly menace be put in place. The 2010 UN General Assembly emphasised that access to water and sanitation are basic human rights requirements. But water which is the number one liquid for life has come under intense pressure, owing to climate change, population explosion, urbanization and scarcity of water in many places. According to WHO, about 50% of the world’s population would be living in water-stressed areas in 2025 [1].
Water quality can be compromised by the presence of unwanted chemicals, micro-organisms and even radiological hazards. The problem of provision of good quality water for human consumption in Nigeria has been a major challenge that has received little or no attention. The National Agency for Food and Drug Administration and Control, (NAFDAC) is the body charged with the responsibility of ensuring the provision of good quality drinking water through the registration and quality assurance of commercially available drinking water [2]. However, majority of the Nigerian populace, in particular students shun commercially available water possibly due to the cost implication and still resort to water sources that lack quality assurance.
The vital role water plays include its ability to dissolve a wide range of substances, and has gained the status of being tagged the ‘universal solvent’. In the human body, two-thirds of the body is made up of water; which is the basic component of cells, tissues and the circulatory system. Due to the solvation character of water, cells are able to access nutrients in the body to produce energy, undergo metabolism and excrete waste in the body. Similarly, for drugs taken to elicit their desired activities, the drug substances must first be dissolved, prior to absorption into systemic circulation. It is well-known that acute dehydration may lead to death, which underscores the role of water as a life-sustaining fluid of great value and importance.
The University of Port Harcourt is sited in Choba community, Obio/Akpor Local Government Area of Rivers state, Nigeria. The state is one of the South-south states that constitute the oil-rich Niger-Delta Area, which has been the subject of oil exploration for more than 50 years. During this time, there have been oil spillages in the environment resulting in air, soil and water pollutions. This is evidenced in the recent United Nations Environment Programme (UNEP) report on the effects of oil spillages in Ogoniland in Rivers state. In this report water samples were obtained from boreholes drilled specifically for the research. The findings from the research revealed high levels of hydrocarbon, some organic and inorganic substances, some of which were carcinogenic [3]. The results further showed that in many locations, petroleum hydrocarbons had migrated to the groundwater. Furthermore, the host community of the University has also played host to an American oil exploration company for over two decades. To this end, it is expected that both soil and water in and around the community will be contaminated, especially with hydrocarbons and heavy metals.
This research aims to determine the physico-chemical parameters and the mineral content of the water sourced from deep water table within the students’ residential area and environs of the main campus of the University of Port Harcourt and to ascertain if the contamination is within safe limits. The standards by which this research would judge water quality is that prescribed by the World Health Organization (WHO), the United States Environmental Protection Agency (EPA) and the Nigerian Industrial Standard developed by the Standards Organization of Nigeria (SON).
Materials and Methods
1.1. Materials
1.1.1. Water Samples
Drinking water samples were collected from students’ residential areas and environs at the University of Port Harcourt Main Campus (Unipark, Abuja); the samples were collected from seventeen locations, which were described in (Table 1). The samples were collected using 2 L glass bottles fitted with an inner cork and an outer screw cap. The bottles were initially washed with detergent, rinsed thoroughly with tap water and then rinsed with distilled water. Prior to sample collection, the bottle was rinsed three times with the sample to be collected before collection. The samples were stored at room temperature. All titrations carried out in the physico-chemical analysis were done in triplicate for each sample and the average titre calculated.
1.2. Methods
1.2.1. pH Determination
Apparatus: pH Meter.
The pH meter was calibrated with standardized solutions of pH 4.0 and 9.1 respectively. The pH was read after inserting the electrode of the pH meter into the sample and allowing the reading to stabilize.
1.2.2. Total Alkalinity
1.2.3. Apparatus/Reagents: Burette, pipette, conical flasks, 0.001105 M HC1, phenolphthalein indicator, and methyl orange indicator.25 ml of the sample was pipetted into a conical flask and 2 drops of phenolphthalein indicator was added. There was no colour change (indicating the absence of carbonate and hydroxyl alkalinity). 2 drops of methyl orange indicator was added to the sample and titrated with the acid to a yellow endpoint.
1.2.4. Calculation:
Total Alkalinity (mg CaCO_3/L) =(M x V x 50000)/V_ (sample ) Bicarbonate Alkalinity (mg CaCO_3/L)=(M x V x 30500)/V_(sample )
Where M= molarity of HCI, V= titre value, and Vsample= Volume of Sample
1.2.5. Dissolved Co2 Content
Apparatus/Reagents: Burette, pipette, conical flasks, 0.01 M NaOH, phenolphthalein indicator.
25 ml of the sample was pipetted into a conical flask and 2 drops of phenolphthalein indicator was added. Titration was done against the base. Endpoint was determined by colour change from colourless to pink.
Calculation
Dissoved CO_2 (mg/L)=(V x N x E x 1000)/V_(sample )
Where V=titre value , N=normality of the base (0.0128), E=equivalent
Weight of co2(22),Vsample=Volume of Sample
1.2.6. Chloride Determination (Precipitation Titration)
Principle:
The principle behind this titration is the precipitation of C1 as AgCl by AgNO3 before AgCrO4 (red) is formed at the endpoint
Apparatus/Reagents: Burette, pipette, conical flasks, 0.014N AgNO3 and K2CrO4 indicator
25 ml of sample was pipetted into a conical flask, 2 drops of the indicator was added and this was titrated against AgNO3 solution until there was a colour change form yellow to brick red.
Calculation:
Chloride (mg/L) =(V x N x E x 1000)/V_(sample )
Where V= titre value, N= normality of AgNO3 (0.014), E= equivalent
Weight of chloride ion (35.5),Vsample=Volume of sample used
1.2.7. Silica Determination (Molybdosilicate Method)
Principle
The Molybdosilicate Method is based on the principle that at a pH of about 1.2, ammonium molybdate ((NH4)6M07024.4H20) reacts with any silica and phosphate present in a sample to form hetero-polyacids. Oxalic acid is then added no neutralize any molybdophosphoric acid present. This reaction produces a yellow colour whose intensity is proportional to the silica that reacted with the molybdate. Standard colour solutions of silica are also prepared and the colour intensity can be visually compared or its absorbance can be measured.
Apparatus: Conical flasks, beakers, pipettes, ammonium molybdate reagent: (NH4)6MO7O24.4H2O), 1:1 HCI, oxalic acid (H2C204.2H20)
Ammonium molybdate: prepared by dissolving 10g of (NH4)6M07024.4H20) in distilled water.
Oxalic acid: prepared by dissolving 7.5 g of H2C204.2H20 in 100 ml of distilled water.
Potassium Chromate (K2CrO4) Solution: prepared by dissolving 315 mg of K2CrO4 in distilled water and made up to 500 ml.
Borax Solution: prepared by dissolving 2.5 g of borate decahydrate Na2B407.10H20 in distilled water and made up to 250 ml.
The standard colour solution of concentrations 0.00 — 1.00 (mg Si/L) was prepared by mixing volumes of distilled water, potassium chromate and borax in the proportion given in (Table 2).
The absorbance of the standard was measured using a UV spectrophotometer at 390 nm. 50 ml of sample was pipetted into a beaker and 2 ml of ammonium molybdate and 1 ml of 1:1 HC1 were added to the beaker. The resulting solution was thoroughly mixed and allowed to stand for 7 minutes. 2 ml of oxalic acid was then added and after 2 minutes, the absorbance of the solution was measured at 390 nm.
Calculation:
The silica content of each sample was determined by means of simple proportion, using the formula:
(Absorbance of standard)/(concentration of silica in standard )=(Absorbance of sample)/(concentration of silica in sample )
1.2.8. Total Hardness Determination (Edta Titrimetric Method)
Principle
Ethylene Diaminetetraacetic Acid, (EDTA) and its sodium salt forms chelated soluble complex when added to a solution of certain metal cations. The addition of a small amount of a dye such as Eriochrome Black T to an aqueous solution containing calcium and magnesium ions at pH of about 10, results in a wine red coloured solution. If EDTA is added as a titrant, any magnesium or calcium will be complexed and the solution will turn from wine red to blue.
Apparatus/Reagents: Burette, pipette, conical flasks, 0.01 M EDTA, Ammonia buffer, Eriochrome Black T indicator. 50 ml of sample was pipetted into the conical flask and 5 drops of indicator was added. 20 ml of Ammonia buffer was added and the resulting mixture was titrated with 0.01 M EDTA solution. The endpoint was determined by a colour change from wine red to blue.
Calculation
Total Hardness (mgCaCO_3/L)=(V x M x E x 2.5 x 1000)/V_sample
Where V=titre value,M=concentration of EDTA,2.5= (molecular mass of Ca〖CO〗_3)/(atomic mass of Ca^(2+) )
E=equivalent weight of Ca^(2+) (40),and V_ sample=Volume of sample
1.2.9. Sulphate Determination (Turbidimetric Method)
Principle:
Sulphate ion is precipitated in a hydrochloric acid medium with barium chloride (BaCI2) to form barium sulphate (BaSO4) crystals of uniform size. The absorbance of the BaSO4 suspension is measured using a UV spectrophotometer and the sulphate ion concentration is determined from the calibration curved developed
Apparatus: UV spectrophotometer, conical flasks, pipettes, beakers, spatula, sulphate conditioning reagent, sulphate stock solution.
Preparation Of Conditioning Reagent: the conditioning reagent was prepared by mixing 45 g of NaCI, 18 ml of conc. HCI, 60 ml of 20 % isopropyl alcohol, 30 ml of glycerol and 180 ml of distilled water in a beaker and stirred thoroughly with a glass rod until the solution was clear. Preparation of Sulphate Stock Solution: this was prepared by dissolving 147.9 mg of anhydrous sodium sulphate (Na2SO4) in 1000 ml of distilled water. Preparation of Sulphate Standard Solution: 0.1, 0.2, 0.3, 0.4 and 0.5 ml respectively of the stock solution was pipetted into five 100 ml volumetric flasks and made up to the 100 ml mark with distilled water to produce 1, 2, 3, 4 and 5 ppm of the sulphate stock solution. These were then transferred into appropriately labelled stopper reagent bottles.
Formation Of Baso4 Turbidity: 5 ml of the conditioning reagent was added to the each of the 100 ml standard solution as well as to 100 ml of each sample. This was stirred for one minute. During stirring, a spatula full of BaCl2 crystals was added. The absorbance or each standard as well as each sample was measured using the UV spectrophotometer at 420 nm. The agitated samples were allowed to stand the in UV spectrophotometer for 4 minutes before recording the reading.
Calculation
The absorbance of the five standard solutions were plotted against their concentrations to obtain a calibration curve. The equation of the resulting curve (Equation 1) was used to calculate the sulphate ion content for each sample.
y = 0.0054x + 0 ----------(equation 1)
(R2 = 0.971)
Where y = sulphate ion content (mg/L), 0.0054 = slope, 0 = intercept, R2 = extent of linearity
1.2.10. Nitrate Determination (Brucine Colorimetric Method)
Apparatus/Reagents: UV Spectrophotometer, volumetric flasks, pipettes, beakers, brucine sulphanilic acid (brucine), conc. H2S04, 30 % NaC1, conc. HNO3, stock nitrate solution.
Preparation of Nitric Acid Stock Solution: 8.5 ml of conc. HNO3 was dissolved in distilled water and diluted to 500 ml in a 1000 ml measuring cylinder.
Preparation of Nitrate Standard Solution: 0.1, 0.2, 0.3, 0.4 and 0.5 ml respectively of the stock solution was pipetted into five 100 ml measuring cylinders and made up to the 100 ml mark with distilled water to produce 1, 2, 3, 4 and 5 ppm of the nitrate stock solution. These were then transferred into appropriately labelled conical flasks.
5 ml of the 1 ppm standard solution was pipetted into a volumetric flask. I ml of 30 % NaCI and 10 ml of conc. H2S04 was added gently to the 1 ppm solution, followed by the addition of 0.1 g of brucine. Upon mixing, a deep red colour which turned yellow was produced. The absorbance of the resulting solution was measured using a UV spectrophotometer at 410 nm. The above procedure was repeated using 5 ml each of the remaining as well as for each sample.
Calculation:
The absorbance of each of five standard solutions was plotted against their concentration to obtain a calibration curve. The equation of the resulting curve (Equation 2) was used to calculate the content for each sample.
y = 0.0038x + 0 ----------------- (Equation 2)
(R2=0.9747)
Where y = nitrate content (mg/L), 0.0038 = slope, 0 = intercept, R2 = extent of linearity
1.2.11. Determination of Calcium, Iron, Zinc, Lead,Chromium, Cadmium And Sodium Content by Atomic Adsorption Spectroscopy
The levels of the above mentioned heavy metals and non-heavy metals were determined using the atomic adsorption spectrometer of the following model: Bulk Scientific 205 AAA Model 210 VGP (with air-acetylene flame on absorbance mode and with injection volume of 7 ml/min). Calcium was determined at a wavelength of 423 nm, sodium at 589 nm, iron at 248, zinc at 214 nm, chromium 357nm, cadmium at 228 nm and lead at 283 nm.
Standard metal solutions for each metal were prepared and calibration curves for each metal were obtained from a linear plot of the absorbance of the standard against their concentrations in mg/L. This was used to determine the concentration of each metal in each sample by extrapolation from the calibration curves. The instrument was first calibrated to zero by aspirating a blank solution in the nebulizer. The samples were then aspirated in the nebulizer at 7 ml/min and the absorbance of each sample recorded.

Where M= molarity of HCI, V= titre value, and Vsample= Volume of Sample
Table 1: Sample sources and description in student’s residential areas and environs.

1.2.5. Dissolved Co2 Content
Apparatus/Reagents: Burette, pipette, conical flasks, 0.01 M NaOH, phenolphthalein indicator.
25 ml of the sample was pipetted into a conical flask and 2 drops of phenolphthalein indicator was added. Titration was done against the base. Endpoint was determined by colour change from colourless to pink.
Calculation

Where V=titre value , N=normality of the base (0.0128), E=equivalent
Weight of co2(22),Vsample=Volume of Sample
1.2.6. Chloride Determination (Precipitation Titration)
Principle:
The principle behind this titration is the precipitation of C1 as AgCl by AgNO3 before AgCrO4 (red) is formed at the endpoint
Apparatus/Reagents: Burette, pipette, conical flasks, 0.014N AgNO3 and K2CrO4 indicator
25 ml of sample was pipetted into a conical flask, 2 drops of the indicator was added and this was titrated against AgNO3 solution until there was a colour change form yellow to brick red.
Calculation:

Where V= titre value, N= normality of AgNO3 (0.014), E= equivalent
Weight of chloride ion (35.5),Vsample=Volume of sample used
1.2.7. Silica Determination (Molybdosilicate Method)
Principle
The Molybdosilicate Method is based on the principle that at a pH of about 1.2, ammonium molybdate ((NH4)6M07024.4H20) reacts with any silica and phosphate present in a sample to form hetero-polyacids. Oxalic acid is then added no neutralize any molybdophosphoric acid present. This reaction produces a yellow colour whose intensity is proportional to the silica that reacted with the molybdate. Standard colour solutions of silica are also prepared and the colour intensity can be visually compared or its absorbance can be measured.
Apparatus: Conical flasks, beakers, pipettes, ammonium molybdate reagent: (NH4)6MO7O24.4H2O), 1:1 HCI, oxalic acid (H2C204.2H20)
Ammonium molybdate: prepared by dissolving 10g of (NH4)6M07024.4H20) in distilled water.
Oxalic acid: prepared by dissolving 7.5 g of H2C204.2H20 in 100 ml of distilled water.
Potassium Chromate (K2CrO4) Solution: prepared by dissolving 315 mg of K2CrO4 in distilled water and made up to 500 ml.
Borax Solution: prepared by dissolving 2.5 g of borate decahydrate Na2B407.10H20 in distilled water and made up to 250 ml.
The standard colour solution of concentrations 0.00 — 1.00 (mg Si/L) was prepared by mixing volumes of distilled water, potassium chromate and borax in the proportion given in (Table 2).
The absorbance of the standard was measured using a UV spectrophotometer at 390 nm. 50 ml of sample was pipetted into a beaker and 2 ml of ammonium molybdate and 1 ml of 1:1 HC1 were added to the beaker. The resulting solution was thoroughly mixed and allowed to stand for 7 minutes. 2 ml of oxalic acid was then added and after 2 minutes, the absorbance of the solution was measured at 390 nm.
Calculation:
The silica content of each sample was determined by means of simple proportion, using the formula:
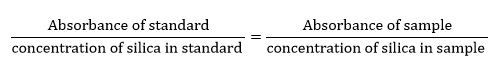
Table 3: Physico-chemical characteristics of the sampled water sources.

1.2.8. Total Hardness Determination (Edta Titrimetric Method)
Principle
Ethylene Diaminetetraacetic Acid, (EDTA) and its sodium salt forms chelated soluble complex when added to a solution of certain metal cations. The addition of a small amount of a dye such as Eriochrome Black T to an aqueous solution containing calcium and magnesium ions at pH of about 10, results in a wine red coloured solution. If EDTA is added as a titrant, any magnesium or calcium will be complexed and the solution will turn from wine red to blue.
Apparatus/Reagents: Burette, pipette, conical flasks, 0.01 M EDTA, Ammonia buffer, Eriochrome Black T indicator. 50 ml of sample was pipetted into the conical flask and 5 drops of indicator was added. 20 ml of Ammonia buffer was added and the resulting mixture was titrated with 0.01 M EDTA solution. The endpoint was determined by a colour change from wine red to blue.
Calculation

1.2.9. Sulphate Determination (Turbidimetric Method)
Principle:
Sulphate ion is precipitated in a hydrochloric acid medium with barium chloride (BaCI2) to form barium sulphate (BaSO4) crystals of uniform size. The absorbance of the BaSO4 suspension is measured using a UV spectrophotometer and the sulphate ion concentration is determined from the calibration curved developed
Apparatus: UV spectrophotometer, conical flasks, pipettes, beakers, spatula, sulphate conditioning reagent, sulphate stock solution.
Preparation Of Conditioning Reagent: the conditioning reagent was prepared by mixing 45 g of NaCI, 18 ml of conc. HCI, 60 ml of 20 % isopropyl alcohol, 30 ml of glycerol and 180 ml of distilled water in a beaker and stirred thoroughly with a glass rod until the solution was clear. Preparation of Sulphate Stock Solution: this was prepared by dissolving 147.9 mg of anhydrous sodium sulphate (Na2SO4) in 1000 ml of distilled water. Preparation of Sulphate Standard Solution: 0.1, 0.2, 0.3, 0.4 and 0.5 ml respectively of the stock solution was pipetted into five 100 ml volumetric flasks and made up to the 100 ml mark with distilled water to produce 1, 2, 3, 4 and 5 ppm of the sulphate stock solution. These were then transferred into appropriately labelled stopper reagent bottles.
Formation Of Baso4 Turbidity: 5 ml of the conditioning reagent was added to the each of the 100 ml standard solution as well as to 100 ml of each sample. This was stirred for one minute. During stirring, a spatula full of BaCl2 crystals was added. The absorbance or each standard as well as each sample was measured using the UV spectrophotometer at 420 nm. The agitated samples were allowed to stand the in UV spectrophotometer for 4 minutes before recording the reading.
Calculation
The absorbance of the five standard solutions were plotted against their concentrations to obtain a calibration curve. The equation of the resulting curve (Equation 1) was used to calculate the sulphate ion content for each sample.
y = 0.0054x + 0 ----------(equation 1)
(R2 = 0.971)
Where y = sulphate ion content (mg/L), 0.0054 = slope, 0 = intercept, R2 = extent of linearity
1.2.10. Nitrate Determination (Brucine Colorimetric Method)
Apparatus/Reagents: UV Spectrophotometer, volumetric flasks, pipettes, beakers, brucine sulphanilic acid (brucine), conc. H2S04, 30 % NaC1, conc. HNO3, stock nitrate solution.
Preparation of Nitric Acid Stock Solution: 8.5 ml of conc. HNO3 was dissolved in distilled water and diluted to 500 ml in a 1000 ml measuring cylinder.
Preparation of Nitrate Standard Solution: 0.1, 0.2, 0.3, 0.4 and 0.5 ml respectively of the stock solution was pipetted into five 100 ml measuring cylinders and made up to the 100 ml mark with distilled water to produce 1, 2, 3, 4 and 5 ppm of the nitrate stock solution. These were then transferred into appropriately labelled conical flasks.
5 ml of the 1 ppm standard solution was pipetted into a volumetric flask. I ml of 30 % NaCI and 10 ml of conc. H2S04 was added gently to the 1 ppm solution, followed by the addition of 0.1 g of brucine. Upon mixing, a deep red colour which turned yellow was produced. The absorbance of the resulting solution was measured using a UV spectrophotometer at 410 nm. The above procedure was repeated using 5 ml each of the remaining as well as for each sample.
Calculation:
The absorbance of each of five standard solutions was plotted against their concentration to obtain a calibration curve. The equation of the resulting curve (Equation 2) was used to calculate the content for each sample.
y = 0.0038x + 0 ----------------- (Equation 2)
(R2=0.9747)
Where y = nitrate content (mg/L), 0.0038 = slope, 0 = intercept, R2 = extent of linearity
1.2.11. Determination of Calcium, Iron, Zinc, Lead,Chromium, Cadmium And Sodium Content by Atomic Adsorption Spectroscopy
The levels of the above mentioned heavy metals and non-heavy metals were determined using the atomic adsorption spectrometer of the following model: Bulk Scientific 205 AAA Model 210 VGP (with air-acetylene flame on absorbance mode and with injection volume of 7 ml/min). Calcium was determined at a wavelength of 423 nm, sodium at 589 nm, iron at 248, zinc at 214 nm, chromium 357nm, cadmium at 228 nm and lead at 283 nm.
Standard metal solutions for each metal were prepared and calibration curves for each metal were obtained from a linear plot of the absorbance of the standard against their concentrations in mg/L. This was used to determine the concentration of each metal in each sample by extrapolation from the calibration curves. The instrument was first calibrated to zero by aspirating a blank solution in the nebulizer. The samples were then aspirated in the nebulizer at 7 ml/min and the absorbance of each sample recorded.
Results and Discussions
The results of the Physico-chemical characteristics of the sampled water sources are presented in (Table 3) below. From the results, the samples can be classified as generally soft. The highest hardness value from the result was 14.67 ± 0.00. According to the Twort Hardness classification, this falls in the soft water category [4]. This is directly related to the calcium levels of the samples. Calcium accounts for about two-thirds of water hardness. The recommended upper limit of calcium in drinking water is 50 mg/L. The calcium values were all less than 6.0 mg/L and this reflected in the low hardness values obtained.
The pH values of all samples were not within the acceptable limit of pH for safe drinking-water. The pH values of all the samples were generally acidic with a range of 4.44 to 6.06. Samples 3, 4, 5, 7, 8, 10, 12 and 17 all had values below 5.0, with sample 12 having the lowest value of 4.44. The acidic nature of most samples can be attributed to the low hardness (soft water) of the samples. Soft water is known to be acidic and this increases the ‘plumbosolvency’ of such water.
Dissolved CO2 is one of the components of carbonate equilibrium in water. The highest value of CO2 was 12.02 ± 1.50 mg/L. Dissolved CO2 is significant in that high values of it (usually above 10 mg/L for surface waters) indicates a significant biological oxidation of the organic matter in water. Dissolved CO2 also has a direct relationship with pH and alkalinity. From the results, the dissolved CO2 level is low for all samples, indicating little biological oxidation of organic matter. At pH values between 4.6 and 8.3, bicarbonate alkalinity is in equilibrium with dissolved CO2. The generally low values of dissolved CO2 corresponds therefore to the generally low (bicarbonate) alkalinity.
Chloride in water does not have a negative health impact. Its impact is aesthetic in nature, with high concentrations exceeding 250 mg/L producing a salty taste (when the associated cation is sodium). The chloride levels of all samples were quite low, the highest value being 66.28 ± 1.33 mg/L.
The silica and sulphate concentrations were very low. The limits are 1-30 mg/L and 250 mg/L, respectively [5]. The silica content was almost insignificant (all less than 0.1 mg/L). The sulphate content was also very low; the highest being 2.96 mg/L for sample 14, and in some cases not determinable (samples. 11 and 15). Nitrate is naturally present in soil, water and food due to the nitrogen cycle. The activities of man also add to increase the nitrate levels in the environment. To this end, WHO and NIS set a limit of 50 mg/L, while EPA stipulates a stricter standard of not more than 10 mg/L (nitrate as nitrogen). The range of nitrate concentration for the samples was 11.32 — 58.68 mg/L by WHO and NIS [6].
Standard samples 13 and 14 have excess of nitrate (58.68 and 52.11 mg/L respectively). The nitrate concentration of sample 12 is just at the threshold (50 mg/L). Nitrate levels can become dangerously increased with the increased use of nitrogen based fertilizers and manure, coupled with the fact that nitrate is extremely soluble. The environment around the boreholes are such that support thriving of bacteria which play a significant role in the nitrogen cycle. Nitrogen easily leaches into groundwater from runoff [7]. Since the sample area is inhabited by mainly adults, the most lethal health effect of nitrate poisoning is not expected to be seen (infants are much more sensitive than adults to methaemoglobinaemia caused by nitrate, and essentially most deaths due to nitrate poisoning have been in infants). However, long term exposure to nitrates can, apart from causing methaemoglobinaemia and anaemia, cause diuresis, starchy deposits and haemorrhaging of the spleen. Nitrites in the stomach can react with food proteins to form nitrosoamines; these compounds can also be produced when meat containing nitrites or nitrates is cooked, particularly using high heat. While these compounds are carcinogenic in test animals, evidence is inconclusive regarding their potential to cause cancer (such as stomach cancer) in humans. The Levels of some selected heavy and non-heavy metals in the water samples were determined and the results shown in (Table 4).
The AAS determination of heavy and non-heavy metals showed that the samples were free from these metals except for sodium and calcium. The range of values for sodium was 0.40 — 16.30 mg/L, well below the guideline value set at 50 mg/L for sodium [8]. Sample 17 was the only sample with a trace of zinc (0.13 mg/L) and this was well below the limit of 3 mg/L set by NIS [9] and 5 mg/L set by EPA [10] The increased corrosivity of these samples therefore has an increased associated risk of dissolving metals and non metals including lead, iron, zinc, nickel, brass, copper and cement/concrete [8]. If the water distribution system was laid with pipes containing any of these metals, then the risk of increased levels of these, especially lead would be high. However, this seems not to be the case because the lead levels obtained from AAS analysis of all the samples were all either zero or very low.
Table 3: Physico-chemical characteristics of the sampled water sources.

Table 4: Levels of some selected heavy and non-heavy metals in water samples.

Conclusion And Recommendation
The physico-chemical analyses performed on the samples, demonstrated that the physico-chemical quality of the water samples were mostly within the specified limits as stated by WHO and EPA. The health implications of the physico-chemical quality were considered to be of importance on the longterm basis, since these contaminants at the levels at which they occurred in the water samples can accumulate over time. The pH of the samples was found to be acidic. It can be concluded that the same acidic aquifer serves the entire sample area. The pH of water must be controlled through increasing alkalinity and calcium levels since acidic water tends to be corrosive and can dissolve metal fittings and cement into water, leading to contamination. Also, the nature of construction materials that have been used and that will be used in the future should be reviewed to ensure that it can withstand the acidity of the water. It was not in the scope of this research to determine the size of the underground water aquifer, but it is recommended therefore that the size of the underground aquifer be determined in other to ascertain the extent to which the recommendations for remediation proposed herein would be implemented. The nitrate levels of 2 samples were also found to exceed the acceptable limit (50 mg/L as nitrate ion), while one sample had 50 mg/L as its value. It is recommended that biological denitrification for surface water and ion exchange for ground water is employed in order to reduce the nitrate levels.
Conflict of Interest
The authors have no conflict of interest to declare.
More information regarding this Article visit: OAJBGSR
https://biogenericpublishers.com/pdf/JBGSR.MS.ID.00151.pdf
https://biogenericpublishers.com/jbgsr.ms.id.00151.text/
For more open access journals click on https://biogenericpublishers.com/

No comments:
Post a Comment