Short Course Digoxin in Acute Heart Failure by Nouira Semir in Open Access Journal of Biogeneric Science and Research
ABSTRACT
Background
Despite many critical voices regarding its efficacy and safety, digoxin may still have a role in the management of heart failure. The objective of this study was to evaluate the efficacy and safety of a short course digoxin therapy started in the emergency department based on clinical outcome after 30 days post hospital discharge.
Methods
From Great Tunisian registry, acute decompensated heart failure (ADHF) patients from January 2016 to January 2018 were identified. Patients with incomplete data were excluded. Digoxin treated and non-treated patients were compared in a matched control study with respect to primary outcomes of all-cause mortality and HF readmission. Secondary outcomes included changes of cardiac output (CO) and left ventricular ejection fraction (LVEF) after 72 hours of hospital admission.
Results
The study population comprised 104 digoxin treated and 229 matched non-treated with a median age of 67.4±12.8. After 72 hours of ED admission, there was a larger increase of CO (17.8 % vs 14%; p=0.015) and LVEF (14.4% vs 3.5%; p=0.003) in digoxin group compared to control group. At 30-day post-hospital discharge 34 (10.2%) patients died and 72 (21.6%) patients were readmitted. Use of digoxin was associated with decreased risk of death and hospital readmission [odds ratio, 0.79 (95% CI, 0.71-0.89)].
Conclusion
In ADHF patients, treatment with digoxin was associated with a significant decrease risk of 30-day mortality and hospital readmission with an improvement of cardiac output and left ventricular ejection fraction.
Key words: Acute heart failure; digoxin; mortality; rehospitalization; emergency department.
INTRODUCTION
Heart failure (HF) is a major worldwide health problem and one of the most important causes of hospital admissions [1,2]. These hospitalizations are responsible for an important economic burden and are associated with high mortality rates, up to 20% following hospital discharge [3,4]. Acute decompensated HF (ADHF) management is difficult given the heterogeneity of the patient population, incomplete understanding of its pathophysiology and lack of evidence-based guidelines. Although the majority of patients with ADHF appear to respond well to initial therapies consisting of loop diuretics and vasoactive agents, these first line treatments failed to decrease post-discharge mortality and readmission rate [5,6]. Investigations of novel therapies such as serelaxin did not show a significant clinical benefit. In a recent multicenter, double-blind, placebo-controlled trial including patients who were hospitalized for acute heart failure, it was shown that the risks of death at 180 days were not lower in patients who had received intravenous serelaxin for 48 hours than in patients who had received placebo [7]. Numerous other clinical trials have been published on ADHF treatment and their results were disappointing in term of efficacy and/or safety [8-11]. Digoxin is one of the oldest compounds in cardiovascular medicine but its beneficial effect is very controversial [12]. Yet, digoxin has many potential beneficial properties for heart failure as it is the only oral inotrope available that did not alter blood pressure neither renal function. Despite its useful hemodynamic, neurohormonal, and electrophysiological effects in patients with chronic congestive HF, concerns about digoxin safety were constantly highlighted [13]. Consequently, the use of digoxin has decreased considerably, in the last 15 years [12]. Digoxin under prescribing is problematic for several reasons. First, it underestimated the substantial beneficial effect of digoxin on the reduction of hospital admissions in HF patients. Second, for its low cost, the favorable cost-effectiveness ratio of digoxin is highly desirable in low-income countries. Moreover, the question whether a short course of digoxin is useful in ADHF was not previously investigated in the era of new heart failure therapies including β-blockers, angiotensin converting enzyme inhibitors and angiotensin-receptor blockers [12]. The objective of this study is to assess the efficacy and safety of a short course digoxin in patients admitted to the ED with ADHF (Figure1 and Figure 2).
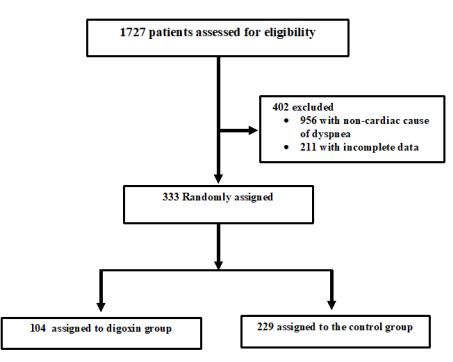
Figure1 : Patients inclusion/exclusion Flowchart
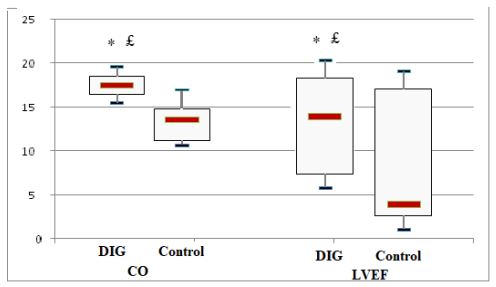
£p=0.015 vs baseline, * p=0.003 vs control group.
Figure 2 : Cardiac output (CO) and left ventricular ejection fraction (LVEF) change from baseline at 72 hours in digoxin (DIG) and control groups.
PATIENTS AND METHODS
Data Source
We conducted a retrospective matched case-control study to assess the association between digoxin treatment and 30-day outcome in patients with ADHF. The ADHF patients were identified from the Great Tunisian database between January 2016 and January 2018. The patients included are residents of a community of 500,000 inhabitants in the east of Tunisia, served by 2 university hospitals (Fattouma Bourguiba Monastir, and Sahloul Sousse). ADHF was defined as an acute onset of symptoms within 48 hours preceding presentation, dyspnea at rest or with minimal exertion, evidence of pulmonary congestion at chest radiograph or lung ultrasound, NT-proBNP ≥1400 pg/ml. This electronic medical recording system provided detail of each patient admitted to emergency department (ED) for acute undifferentiated non traumatic dyspnea.
Study Population
Patients were included if the following data are available: demographic characteristics, comorbidities, current drug use, baseline NYHA functional class, physical exam findings, standard laboratory tests, brain natriuretic peptide levels at ED admission; echocardiographic results, bioimpedance measured cardiac output at ED admission and at hospital discharge, digoxin daily dose, 30-day follow-up information including ED readmission and survival status. A patient who received at least 0.25 mg of oral digoxin (1 tablet) for three days during hospital stay was defined as case; those who did not receive digoxin treatment were selected as control. The protocol used in this study was approved by the ethics committee of our institution, and all subjects gave their written informed consent to be included in the data base. All the listed criteria have to be fulfilled for patient’s inclusion. Exclusion criteria included ongoing treatment with digoxin, pregnant or breast-feeding women, patients with known severe or terminal renal failure (eGFR<30 ml/min/1.73m2), alteration of consciousness (Glasgow coma score <15) and patients needing immediate hemodynamic or ventilatory support. Cases were matched first for sex, then for age (±2 years) and NYHA functional class. We performed an individual matching; we matched each patient under digoxin (case) with 2 patients who did not receive digoxin (control) for age, gender and New York Heart Association (NYHA) classification. Reviewers were limited to matching criteria data only (e.g., blinded to 30-day outcomes) to eliminate potential sources of bias. Patients who were treated with digoxin and those who did not receive digoxin were clinically managed the same way.
Outcome Measures
The main end points included death or rehospitalization within 30 days after hospital discharge, and 30-day combined death-rehospitalization outcomes. Secondary end-points included CO change from baseline and length of stay in the hospital during the index episode.
Statistical Analysis
Baseline characteristics were compared between groups to detect any differences between cases and controls; independent t-tests were performed for normally distributed variables; Mann Whitney U tests were performed for continuous non-normally distributed variables; and chi square analyses were performed for categorical variables. Logistic regression analysis was performed to identify the odds ratios (ORs) and 95% confidence intervals (95% CIs) for hospital readmission and/or death risk with respect to digoxin treatment. Data are reported as means ± standard deviations, unless otherwise noted, and a p-value less than 0.05 via two-sided testing was considered statistically significant. Data were analyzed using the statistical software package SPSS version 18.
RESULTS
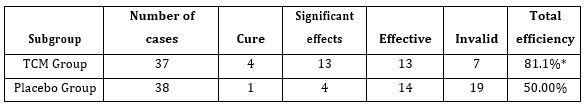
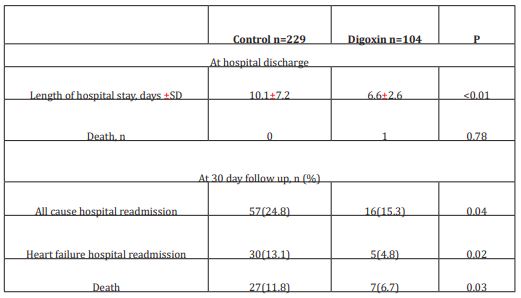
The initial study population comprised 1727 participants who were registered in the database. From this initial population, we excluded 956 with non-cardiac cause of dyspnea, and 211 with incomplete data. From the remaining patients, 104 were included in the digoxin group and 229 in the control group. Digoxin was orally administered once a day and almost all of our patients received the same dose (0.25 mg, one tablet) each day during at least three days. Only few patients received a lower (0.125 mg) or a higher (0.5mg) dose. Baseline characteristics of both groups are shown in table 1. Demographic characteristics were comparable among both study’s groups. There were no relevant differences in age, sex, or NYHA classification. The NYHA class collected was related to base line medical status (within three months before the ongoing exacerbation). Cardiovascular medical history was comparable for both groups. There were no significant differences between cases and controls regarding underlying other comorbidities. Fifty-two percent of the patients had ischaemic cardiomyopathy as the primary aetiology of their heart failure (47-57%) (Table1). Principal baseline medication consisted of diuretics, angiotensin converting enzyme-inhibitors, beta-blockers, and nitrates. Mean vital signs values at baseline were comparable among the 2 groups with respect to heart rate, respiratory rate, and blood pressure. NT-proBNP levels ranged from 1412 to 8615 pg/ml between; 61% in digoxin group and 59% in control group had reduced LVEF (<45%) (p=0.77). After 72 hours of ED admission, there was a larger increase of CO (17.8% vs 14%; p=0.015) and LVEF (14.4% vs 3.5%; p=0.003) in digoxin group compared to control group (Figure 1); NTpro BNP levels decreased and in digoxin group (2%) and in control group (1.2%) but the difference was not significant (p=0.06). Digoxin treatment was associated with a reduced length of hospital stay (10.1±7.2 days versus 6.6± days; p<0.01). At 30-day follow-up, digoxin group showed a significantly lower all-cause (p=0.04) and heart failure (p=0.02) hospital readmission rate compared to control group, and lower mortality (11.8% versus 6.7%; p=0.03) (Table 2). Digoxin treatment was found to significantly decrease the odds for the combined events of mortality and hospital readmission [odds ratio, 0.79 (95% CI, 0.71-0.89)]. No major side effects were observed in relation to digoxin therapy.
Table 1: Baseline characteristics of both study groups
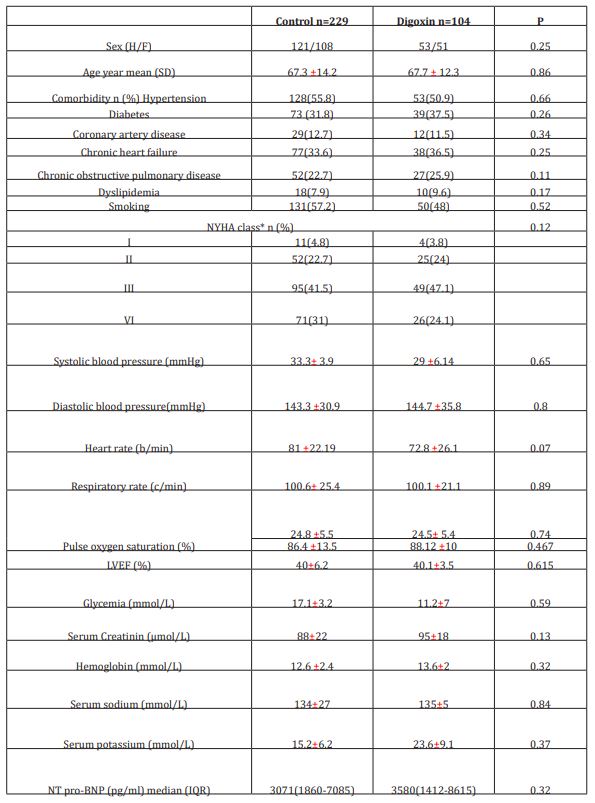
IQR: interquartile range
*NYHA related to base line medical status (within three months before the ongoing exacerbation)
Table 2: Clinical outcomes
DISCUSSION
Our results demonstrated that digoxin is associated with a lower risk of 30-day hospital readmission among ED patients with decompensated HF. Compared with control group, LVEF and cardiac output increased and length of hospital stay decreased significantly in digoxin-treated group. Most available studies analyzed long-term effect of digoxin in patients with chronic heart failure, but data on the effect of short course digoxin on early clinical outcome and physiological related parameters in patients with acute heart failure are scarce. The concordance between physiological and clinical outcomes was in favor of the validity of our results. Digoxin is one of the oldest drugs used in cardiology practice, and few decades ago, it was prescribed in more than 60% of heart failure patients in the United States [14]. Digoxin is the only inotropic drug known to increase cardiac output and to reduce pulmonary capillary pressure without increasing heart rate or lowering blood pressure in contrast to other oral inotropes. However, despite the evidence of its beneficial effects on hemodynamic, neuro-hormonal and electrophysiological parameters, a great concern regarding its safety profile has been raised and the use of digoxin has declined significantly over the past two decades [15]. Indeed, in the ESC guidelines (2016), digoxin indication was limited only to patients with AF and rapid ventricular rate [16]. This could be understandable given the scarcity of randomized trials specifically aimed at testing digoxin safety in heart failure patients. The Digitalis Investigation Group (DIG) trial, the only large randomized trial of digoxin in heart failure, reported a significant reduction in heart failure hospitalizations [17]. Most of the identified studies against the use of digoxin had many potential sources of bias requiring careful assessment. In fact, digoxin safety concern comes from very heterogeneous studies and non-experimental observational studies carrying a high risk of misinterpretation [18-20]. A recent study concluded that prescription of digoxin is an indicator of disease severity and not the cause of worse prognosis which means that a significant prescription bias might be caused by the fact that sicker patients, having a higher mortality risk receive additional treatment with digoxin [21]. Currently, in DIG trial, there is no evidence of an increased risk with digoxin treatment. Importantly, DIG trial demonstrated that beneficial digoxin effects were mainly observed in patients with HFrEF and those with serum digoxin concentration ≤0.9ng/ml. Digoxin efficacy may be attributed in part to the neurohormonal‐inhibiting properties of digoxin, especially in lower doses; it may also be related to its synergistic effects with beta-blockers as pro‐arrhythmic effects of digoxin would be expected to be attenuated by β‐blockers [22].
Our study has several limitations. First, as this is a retrospective analysis, we should clearly highlight that our results only describe associations and not causality. Second, our study is limited by its small sample size. Third, as in all case control studies; bias due to unmeasured confounders remains possible. We should have used the propensity-score matching to better match our two groups but we should point out that most of confounding variables influencing outcome were well balanced between the 2 groups of our study. Third, we had no data regarding post-discharge adherence to prescribed treatment nor we had informations on neither serum digoxin concentration nor the incidence of digoxin toxicity. We acknowledge that this important information would be a valuable support to our findings in demonstrating a correlation between serum digoxin levels and their clinical outcome in our patients. In addition, in our study only 30% of our patients were receiving aldosterone antagonists, and none were receiving cardiac resynchronization therapy, which may limit generalizability of our results.
CONCLUSIONS
Our findings provided an additional data to support the association between use of digoxin and clinical benefit in HF patients with reduced LVEF. Digoxin may potentially serve as an inexpensive tool for the reduction of short-term mortality and hospital readmissions which is an important objective especially in low-income countries in the health system.
More information regarding this Article visit: OAJBGSR
https://biogenericpublishers.com/pdf/JBGSR.MS.ID.00258.pdf
https://biogenericpublishers.com/jbgsr-ms-id-00258-text/